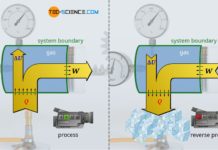
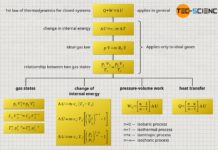
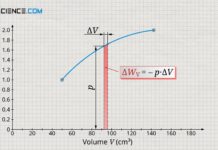
Flow work in open systems
The work which is required to maintain the flow against the different static pressures between inlet and outlet of an open system is referred to as flow work.
Introduction
Many thermodynamic processes take...
Reversibility of thermodynamic processes (entropy)
In thermodynamics, reversible processes are processes which are reversible from an energetic point of view!
Introduction
The free adiabatic expansion of an ideal gas in a vacuum showed that this thermodynamic process cannot...
What is the Joule-Thomson effect?
The Joule-Thomson effect describes the decrease in temperature of real gases when they expand against a lower pressure!
In the article Free expansion of an ideal gas in a vacuum it was...
Free adiabatic expansion of an ideal gas in a vacuum
The expansion of an ideal gas against a vacuum in an adiabatic system is an isothermal process!
In the article on the isentropic process it was already indicated that the often used...
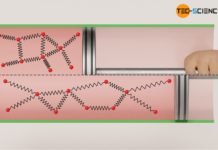
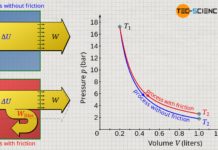
Dissipative thermodynamic processes in adiabatic systems
In this article, learn more about dissipative thermodynamic processes using the polytropic equations.
Work performed in adiabatic systems
Many thermodynamic processes take place within very short times, such as the expansion of the...
Polytropic process in a closed system
Learn more about polytropic thermodynamic processes in closed systems in this article.
Particular processes shown in a volume-pressure diagram
The figure below shows the course of the pressure as a function of the...
Derivation of the formulas for work and heat of a polytropic process
In this article you will learn more about the derivation of the formulas for calculating work, heat and change of internal energy for polytropic processes.
Polytropic equations
In the article Polytropic Process in...
Derivation of the formulas of the isentropic “adiabatic” process
In this article, learn more about the derivation of the formulas and equations describing the isentropic (adiabatic) process.
Basic equations
For the derivation of the equations describing the isentropic process, the first law...
Isentropic (“adiabatic”) process in a closed system
An isentropic process is a reversible process of an adiabatic system.
Definition
Whereas in an isochoric process no pressure-volume work is done by the system or on the system (Wv=0) and in an...
Isothermal process in a closed system
In this article, learn more about the calculation of pressure, volume, work and heat in an isothermal process in a closed system.
A change of state of a gas in which the...
Isobaric process in a closed system
In this article, learn more about the calculation of volume, temperature, work, and heat in an isobaric process in a closed system.
A change of state of a gas in which the...
Isochoric process in a closed system
In this article, learn more about the calculation of pressure, temperature, work and heat in an isochoric process in a closed system.
A change of state of a gas in which the...
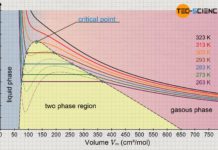
Explanation of liquefaction using the Van der Waals equation
In this article, learn how the Van der Waals equation can be used to explain the liquefaction of gases at high pressures.
Van der Waals equation
The ideal gas law describes the behavior...
What is meant by dissipation of energy?
Dissipation is the (partial) conversion of a certain form of energy into thermal energy that cannot be fully converted back into the original form of energy.
Definition
Dissipation of energy means that when...
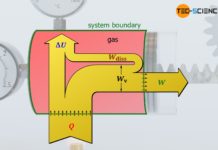
Concept of pressure-volume work (displacement work)
The pressure-volume work (displacement work) is the work don on the gas or by the gas due to the acting gas pressure during a change in volume.
Heat engines and heat pumps
Gases...
Dissipation of energy in closed systems
Learn in this article why, in thermodynamic processes with dissipation of energy, the pressure-volume work of the gas does not correspond to the work done by the system.
Pressure-volume work (displacement work)
In...
Derivation of the pressure-volume work (displacement work)
Learn in this article why the area under the curve in a volume-pressure diagram corresponds to the pressure-volume work (displacement work).
In the article concept of pressure-volume work, it was said without...
Cooling drinks with ice cubes (Derivation and calculation with formula)
Learn more about calculating the final temperature of a drink when cooling with ice cubes in this article.
Introduction (Excel spreadsheet for calculation)
If you want to cool a warm drink relatively quickly...
Why does water boil faster at high altitudes?
Due to the lower pressure, the boiling point of water decreases and the water boils earlier at high altitudes.
https://www.youtube.com/watch?v=-nf8w-SeFDM
Cooking on Mount Everest
With increasing altitude above sea level, the air pressure decreases...
Why do pressure and temperature increase during the compression of a gas?
The energy added as work during the compression of a gas leads to an increase in pressure and temperature. Learn more about this in this article.
While an increase in temperature due...
Difference between latent heat of vaporization and enthalpy of vaporization
For isobaric vaporization, the added heat of vaporization (process quantity) leads to a change in the enthalpy of the substance (state variable).
Increase in volume during vaporization
In the article Specific latent heat...
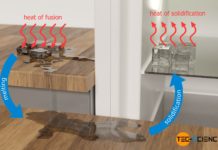
Vineyard Frost Protection (sprinkling with water)
With sprinklers for frost protection, the crop stays protected from low temperatures by the heat of solidification released when the water freezes.
https://www.youtube.com/watch?v=bh43jUpB_F4
If growing fruits are exposed to sub-zero temperatures (frost) on...
Why steam burns are more dangerous than water burns?
Steam burns are more dangerous than water burns because more heat is transferred due to the additional release of latent heat of condensation.
https://www.youtube.com/watch?v=bh43jUpB_F4
To vaporize a liquid, energy as heat must be...
Why does water extinguish fire?
By absorbing a very large amount of heat during vaporization, water draws energy from the fire site and thus cools it down until the fire goes out!
https://www.youtube.com/watch?v=bh43jUpB_F4
The simple answer to this...
Specific latent heat of solidification (enthalpy of solidification)
Specific heat of solidification is the heat energy to be released for solidification of a liquid per kilogram of the substance!
https://www.youtube.com/watch?v=bh43jUpB_F4
Melting and solidification
In the article on specific latent heat of fusion,...
Specific latent heat of fusion (enthalpy of fusion)
The specific latent heat of fusion (enthalpy of fusion) is the amount of heat required to melt a solid substance!
https://www.youtube.com/watch?v=bh43jUpB_F4
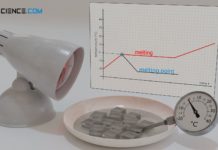
Process of melting
If a solid is heated more and more, then at...
Specific latent heat of condensation
Specific heat of condensation is the heat energy to be released for condensation of a gas per kilogram of the substance!
https://www.youtube.com/watch?v=bh43jUpB_F4
Vaporization and condensation
In the article on specific latent heat of vaporization,...
Specific latent heat of vaporization
The specific latent heat of vaporization (enthalpy of vaporization) is the amount of heat required to vaporize a liquid substance!
https://www.youtube.com/watch?v=bh43jUpB_F4
Process of vaporization
If a liquid is heated more and more, then at...
Van der Waals equation (gas law for real gases)
The Van der Waals equation describes the relationship between pressure, volume and temperature for real gases.
Ideal gas law
In thermodynamic processes, gases are often considered as ideal gases for simplicity. Depending on...
Why does the temperature remain constant during a change of state (phase transition)?
During a change of the state of matter, the supplied energy is not used to increase the kinetic energy of the molecules, but to change the binding energies. Therefore, the temperature...
Final temperature of mixtures (Richmann’s law)
Richmann's law of mixtures describes the final temperature resulting in thermodynamic equilibrium when two bodies with different initial temperatures are brought into contact.
https://youtu.be/6tue9N7M1vU
Adiabatic mixing
If two bodies with different initial temperatures are...
Heating and cooling of several objects
Learn more about calculating the final temperature of several objects with different temperatures in this article.
Heating of several objects
In practice, when heating or cooling objects, one usually has to deal with...
Heat capacity of objects
Heat capacity is the amount of heat required to raise the temperature of an object by 1 Kelvin (1 °C). Learn more about it in this article.
Specific heat capacity of substances
The...
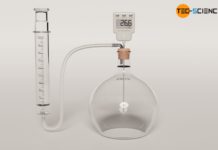
Calorimeter to determine the specific heat capacities of liquids
Calorimetry deals with the measurement of heat energy.These measurements are based on temperature changes, which are used to determine the amount of heat involved.
Test setup
The experimental setups used in calorimetry are...
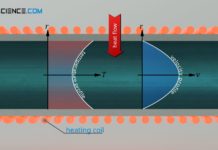
Specific heat capacity of gases (at constant volume or pressure)
Due to compressibility of gases, a distinction must be made between the isobaric and the isochoric specific heat capacity.
https://www.youtube.com/watch?v=0JZOK2hcQok
Differentiation between isochoric and isobaric heat transfer
Unlike liquids or solids, gases are special...
Specific heat capacity of water
The specific heat capacity of water depends on the temperature and is strongly dependent on the state of matter.
https://www.youtube.com/watch?v=0JZOK2hcQok
The specific heat capacity is not a material constant for a substance, but...
Specific heat capacity of selected substances
In this article, learn more about the specific heat capacity of different materials and how it affects the change in temperature over time during a heat transfer.
https://www.youtube.com/watch?v=0JZOK2hcQok
Definition of the specific heat...
Important remarks on the specific heat capacity
https://www.youtube.com/watch?v=0JZOK2hcQok
Definition of the specific heat capacity
The specific heat capacity c describes the relationship between a transfer of heat Q and the associated temperature change ΔT of a substance of mass m:
begin{align}label{q}&...
Specific heat capacity (derivation and definition)
The specific heat capacity indicates how much heat must be absorbed by a substance of mass 1 kg in order to increase its temperature by 1 K (1 °C).
https://www.youtube.com/watch?v=0JZOK2hcQok
Introduction
The temperature of...
Internal energy of ideal gases
In ideal gases, the change in internal energy is directly related to the change in temperature. Learn more about the relationships in this article.
Simplified assumptions for ideal gases
In thermodynamics, gases play...
Calculation of the internal energy for ideal gases
Learn more about calculating the internal energy for ideal gases in this article.
First law of thermodynamics
In the article Internal energy of ideal gases it was explained in detail that in ideal...
Law of Gay-Lussac for ideal gases (Charles’s law)
The law of Gay-Lussac describes the relationship between an increase in temperature and the resulting increase in volume at constant pressure (isobaric process).
Isobaric process
If a thermodynamic process takes place at constant...
Law of Amontons for ideal gases
The law of Amontons describes the relationship between an increase in temperature and the resulting increase in pressure at constant volume (isochoric process).
Isochoric process
If a thermodynamic process takes place at constant...
Law of Boyle-Mariotte for ideal gases
The law of Boyle-Mariotte describes the relationship between a decrease in volume and the resulting increase in pressure at constant temperature (isothermal process).
Isothermal process
If a thermodynamic process takes place at constant...
Why does ice form on the top of a lake?
Learn in this article why ice form always on top of a lake in winter.
The negative thermal expansion of water (density anomaly) has an existential importance for life on earth. More...
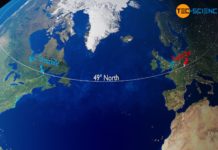
Gulf Stream & global ocean conveyor belt
The Gulf Stream is an ocean current in the Atlantic Ocean which, as part of the earth's global conveyor belt, has a decisive influence on the climate in Northern and Western...
Difference between thermal conductivity, diffusivity, transmittance, resistance and heat transfer coefficient
Learn more in this article about the differences and importance of thermal conductivity, thermal diffusivity, heat transfer coefficient, thermal transmittance and thermal resistance, etc.
Thermal conductivity
Thermal conductivity (lambda) describes the heat transfer...
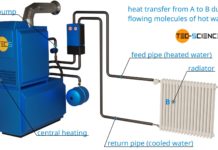
Why are radiators usually located under a window?
Learn in this article, why radiators are usually located under a window?
Central heating systems use the principle of thermal convection. The water is heated by a central heater and then transferred...
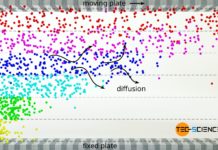
Viscosity of an ideal gas
The viscosity of ideal gases is mainly based on the momentum transfer due to diffusion between the fluid layers.
Definition of viscosity
In the article Viscosity, the cause of viscosity was mainly attributed...
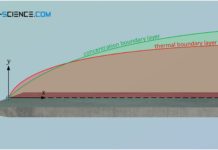
Dimensionless numbers of the boundary layers (Prandtl, Schmidt and Lewis number)
To describe the heat and mass transport, dimensionless numbers are introduced to describe the processes within the boundary layers.
Between a flowing fluid and a solid surface, different boundary layers are formed,...
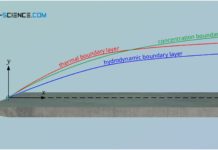
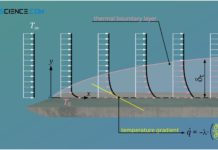
Thermal and concentration boundary layer
In addition to the hydrodynamic boundary layer, the thermal boundary layer and the concentration boundary layer also have a decisive influence on the entire heat and mass transport in a flow.
Temperature...
Prandtl number
The Prandtl number is a dimensionless similarity parameter to describe the transport of heat and momentum.
Definition
In the article on the different boundary layers, the importance of these boundary layers with respect...
Lewis number
The Lewis number is a dimensionless similarity parameter to describe heat and mass transport.
The Lewis number always comes into play when a flowing fluid is transferring both heat by conduction and...
Calculation of the Nusselt numbers for forced flows over plates and in pipes
In this article you will find formulas for calculating the local and average Nusselt numbers for forced flows over plates and in pipes with circular cross sections.
Nusselt number
The definition and importance...
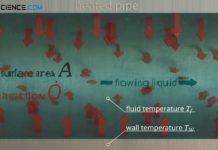
Heat transfer coefficient for thermal convection
The heat transfer coefficient describes the convective heat transfer from a solid to a flowing fluid and vice versa!
Introduction
The heat transfer coefficient describes the convective heat transfer from a solid to...
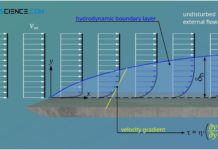
Hydrodynamic boundary layer
The hydrodynamic boundary layer of a flow has a decisive influence on heat and mass transport.
Introduction
In this article we take a closer look at the boundary layers between a solid surface...
Nusselt number to describe convective heat transfer
The Nusselt number is a dimensionless similarity parameter to describe convective heat transfer, independent of the size of the system.
Introduction
Convective heat transfer describes the heat transport between a solid surface and...
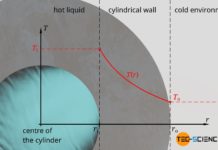
Temperature profiles and heat flows through different geometries
In this article we discuss temperature curves and heat flows through a plane wall, through a cylindrical pipe and through a hollow sphere.
Introduction
Temperature differences cause heat flows. These heat flows, in...
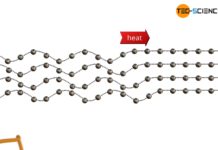
Thermal conduction in solids and ideal gases
The thermal conductivity in crystalline, non-metallic solids first increases and then decreases again with increasing temperature.
Phonons: Quasiparticles of the lattice vibrations
Thermal conduction refers to the transfer of thermal energy through a...
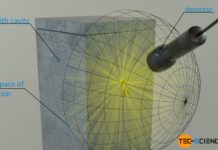
Thermodynamic derivation of the Stefan-Boltzmann Law
In this article the Stefan-Boltzmann-Law is to be derived using the laws of thermodynamics.
https://www.youtube.com/watch?v=oo-A0uGeUlE
Introduction
In this article, the Stefan-Boltzmann Law is to be derived with the laws of thermodynamics. In order to...
Different forms of Planck’s law
Planck's law of radiation can be expressed in different forms. The most important ones are discussed in this article.
https://www.youtube.com/watch?v=JxDgEUsgAIs
Introduction
Planck's law of radiation describes the radiation emitted by black bodies. However, there...
Planck’s law and Wien’s displacement law
Planck's law describes the radiation emitted by black bodies and Wien's displacement law the maximum of the spectral intensity of this radiation.
https://youtu.be/lKoMK30GVrY
Blackbody radiation
The emitted wavelength spectrum of a blackbody as shown...
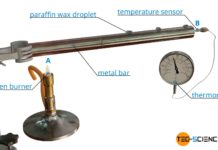
Laser-Flash method for determining thermal conductivity (LFA)
With the Laser-Flash method (Laser Flash Analyser, LFA), the thermal conductivity is determined by the temperature rise in a test sample that is heated by a short laser pulse from one...
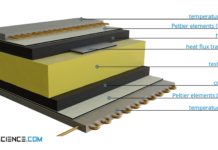
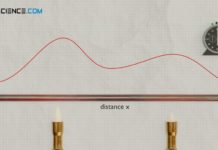
Transient-Hot-Wire method method for determining thermal conductivity (THW)
With the Transient-Hot-Wire method (THW), the thermal conductivity is determined by the change in temperature over time at a certain distance from a heating wire.
Design
With the transient-hot-wire method, the thermal conductivity...
Heat-Flow-Meter method for determining thermal conductivity (HFM)
With the Heat-Flow-Meter method (HFM) the thermal conductivity is determined by comparative measurement of the heat flow using a reference sample.
Thermal conductivity
Thermal conductivity is a measure of how well or poorly...
Guarded-Hot-Plate method for determining thermal conductivity (GHP)
With the Guarded-Hot-Plate method (GHP) the thermal conductivity is determined by the electrical power output of a hot plate with guided heat conduction.
Thermal conductivity
Thermal conductivity is a measure of how well...
Derivation of heat equation (diffusion equation)
The heat equation describes the temporal and spatial behavior of temperature for heat transport by thermal conduction.
Derivation of the heat equation
We first consider the one-dimensional case of heat conduction. This can...
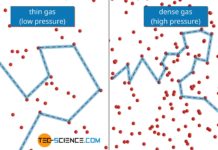
Thermal conductivity of gases
The thermal conductivity of ideal gases is not dependent on pressure for gases that are not too strongly diluted. This is no longer the case for gases with low pressure.
Introduction
In the...
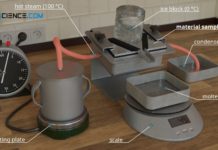
Experimental setup for determining thermal conductivity
In this article you can learn more about the experimental determination of the thermal conductivity of materials using steam and ice.
Thermal conductivity
Thermal conductivity is a measure of how well or poorly...
Thermal transmittance (U-value)
The thermal transmittance (U-value or U-factor) describes the heat transfer through a solid object, which is located between two fluids (gas or liquid) with different temperatures.
Definition and unit of the U-value
The...
How does a thermos work? Design of a vacuum flask!
Learn more about the structure of a vacuum flask and how a thermos works in this article!
The reason why hot tea or coffee stays warm for so long in a thermos...
Heat transfer by thermal radiation
With thermal radiation, heat is transferred by electromagnetic waves without the presence of a substance!
The mechanisms of thermal convection and thermal conduction explained in separate articles have one thing in common:...
Heat transfer by thermal convection
With heat transfer by thermal convection, heat is transported with a flowing substance. Convection only occurs in fluids, i.e. gases and liquids.
Introduction
One possibility of heat transfer is that hot substances flow...
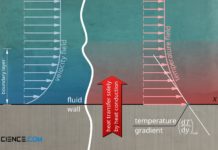
Heat transfer by thermal conduction
Heat transfer by thermal conduction means that heat is conducted through a material. Heat energy is transferred from molecule to molecule at the atomic level.
Introduction
The principle of thermal convection can basically...
Rate of heat flow: Definition and direction
The rate of heat flow refers to the heat energy transferred per unit of time (heat output). The drive for the heat flow is a temperature difference.
Direction of the heat flow
If...
Thermal conductivity (Fourier’s law)
Thermal conductivity is a measure of how well or poorly a material conducts heat energy (measure of the strength of heat conduction)!
Thermal conduction
In general, heat can be transferred in three different...
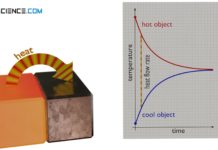
Heat and thermodynamic equilibrium
In thermodynamics, heat is the transport of energy due to a temperature difference. Heat in this respect is never "contained" in an object!
Equalization of temperature of two substances
Everyday experience shows that...
Heat transfer (heat transport)
Heat transfer is the transport of thermal energy from a warmer object to a cooler object. A distinction is made between convection, conduction and radiation.
Example 1: Building
The figure below shows a...
The process quantities: Heat and work
Work and heat are process quantities that describe the process of a supply of energy ("energy in transit")! Learn more about it in this article.
Work and heat transferred to a substance
Energy...
Internal energy & first law of thermodynamics
Internal energy is the sum of the different forms of energy on a microscopic level inside a substance. Learn more about it in this article.
Energy transfer as heat and as work
In...
Thermodynamic systems
A thermodynamic system is a confined space of matter (e.g. gas) within which thermodynamic processes take place. The system boundary separates the system from the environment.
Definition of the term thermodynamic system
In...
Stefan-Boltzmann law & Kirchhoff’s law of thermal radiation
The Stefan-Boltzmann law states that the radiant power of an object in thermal equilibrium is proportional to the fourth power of temperature and directly proportional to its surface!
https://youtu.be/U67D8q88UDA
Introduction
In the article Blackbody...
Internal energy & heat capacity of ideal gases (kinetic theory of gases)
In this article, learn more about the relationship between internal energy and heat capacity in connection with the kinetic theory of gases.
https://www.youtube.com/watch?v=M9i8YfBu30w
Internal energy
In the article equipartition theorem it has already been...
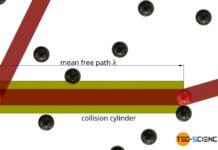
Mean free path & collision frequency (derivation)
The mean free path is the average distance a particle travels without colliding with other particles!
https://www.youtube.com/watch?v=56JxrWySbvg
Introduction
In the article Maxwell-Boltzmann distribution it was shown that the mean speed (average speed) of the...
Derivation of the Maxwell-Boltzmann distribution function
The Maxwell-Boltzmann distribution function of the molecular speed of ideal gases can be derived from the barometric formula.
https://www.youtube.com/watch?v=SPiWzK9I-RI
Introduction
For ideal gases, the distribution function f(v) of the speeds has already been explained...
Equipartition theorem
The equipartition theorem states that the kinetic energy of the gas molecules is equally divided along all three spatial directions!
https://www.youtube.com/watch?v=wxFbzgYe9cM
Equipartition theorem
In the article Pressure and temperature the following equation was derived...
Ideal gas law (explained and derived)
The ideal gas law describes the relationship between pressure, volume, mass and temperature of ideal gases.
Parameters influencing the gas pressure
The figure below shows a gas-filled cylinder closed with a piston. If...
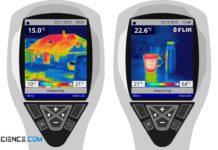
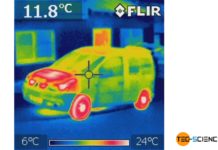
How does a thermal imaging camera work?
Thermal imaging cameras are based on the same principle as the pyrometer. These cameras capture the radiation spectrum of an object, which then allows conclusions to be drawn about the temperature...
How does a infrared thermometer (pyrometer) work?
Pyrometers (infrared thermometers) use the heat radiation of objects invisible to the human eye to determine the temperature!
The thermometers presented in the previous articles must directly touch the object from which...
How does a thermocouple work?
In this article we will deal with the design and operation of a thermocouple which is used to measure temperatures.
https://youtu.be/y7efQiLkfkM
Seebeck effect
A thermocouple uses the phenomenon that a voltage is caused by...
How does a resistance temperature detector (resistance thermometer) work?
In resistance thermometers the dependence of the electrical resistance on the temperature is used to determine the temperature!
https://youtu.be/L5I8_AcJ_nE
Operating principle
A further measuring principle for determining the temperature is the change in the...
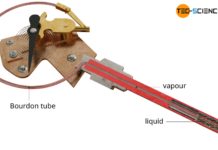
How does a vapor pressure thermometer (vapor-in-metal) work?
Vapor pressure thermometers use the temperature-dependent vapor pressure of a liquid as the measuring principle.
https://youtu.be/K6HYsY6fF8k
The less popular vapour pressure thermometers work according to the same principle as liquid-in-metal thermometers or gas-in-metal...
How does a gas filled thermometer (gas-in-metal) work?
In gas thermometers, the pressure rise connected with a temperature increase is used for measuring the temperature!
https://youtu.be/aYpHDEhvzLo
In gas filled temperature gauges (also called gas thermometers or gas-in-metal thermometers), a gas is...
How does a liquid filled thermometer (liquid-in-metal) work?
Liquid-in-metal thermometers use the pressure rise of a liquid that comes along with an increase in temperature, if the volume is kept constant!
https://youtu.be/Q7YNHcrrg2o
In liquid-in-metal thermometers (also referred to as liquid filled...
How does a bimetallic strip thermometer work?
In bimetallic strip thermometers the different rates of expansion of metals when heated is used to measure the temperature!
https://youtu.be/RFWj7L05Yxo
Operating principle
Temperatures can be meassured based on the principle of different thermal expansion...
How does a liquid-in-glass thermometer work?
In liquid-in-glass thermometers, the thermal expansion of liquids is used for measuring the temperature!
https://youtu.be/4ZcQ1HQbQwo
Operating principle
Liquid-in-glass thermometers are based on the principle of thermal expansion of substances. A liquid in a glass...
Determination of the speed distribution in a gas
Learn more about experimentally determining the velocity distribution of molecules in gases in this article.
https://www.youtube.com/watch?v=OVcen-FWc-4
Introduction
As already explained in the article Temperature and particle motion, the temperature of a gas is a...
Pressure and temperature (kinetic theory of gases)
In this article, learn more about the relationship between pressure and temperature in connection with the kinetic theory of gases.
https://www.youtube.com/watch?v=3NYFD9LMV2A
Introduction
In order to connect the macroscopically observed state variables of a gas...
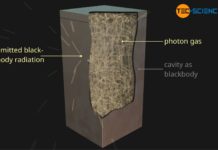
Blackbody radiation
Blackbody radiation (cavity radiation) is the thermal radiation of a blackbody, i.e. a body that absorbs all incident radiation.
https://www.youtube.com/watch?v=Na-mFjyP8eU
Blackbody
When white light hits an opaque object, a certain part of that light...