Derivation of the Maxwell-Boltzmann distribution function
The Maxwell-Boltzmann distribution function of the molecular speed of ideal gases can be derived from the barometric formula.
https://www.youtube.com/watch?v=SPiWzK9I-RI
Introduction
For ideal gases, the distribution function f(v) of the speeds has already been explained...
Equipartition theorem
The equipartition theorem states that the kinetic energy of the gas molecules is equally divided along all three spatial directions!
https://www.youtube.com/watch?v=wxFbzgYe9cM
Equipartition theorem
In the article Pressure and temperature the following equation was derived...
Determination of the speed distribution in a gas
Learn more about experimentally determining the velocity distribution of molecules in gases in this article.
https://www.youtube.com/watch?v=OVcen-FWc-4
Introduction
As already explained in the article Temperature and particle motion, the temperature of a gas is a...
Pressure and temperature (kinetic theory of gases)
In this article, learn more about the relationship between pressure and temperature in connection with the kinetic theory of gases.
https://www.youtube.com/watch?v=3NYFD9LMV2A
Introduction
In order to connect the macroscopically observed state variables of a gas...
Maxwell–Boltzmann distribution
The Maxwell-Boltzmann distribution describes the distribution of the molecular speed of the molecules in ideal gases.
https://www.youtube.com/watch?v=wjflAaq2amg
Introduction
As already explained in the article Temperature and particle motion, the temperature of a gas is...
Why do liquids evaporate?
In this article, learn how the evaporation of liquids can be qualitatively explained using the Maxwell-Boltzmann distribution.
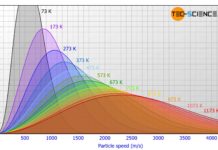
Maxwell-Boltzmann distribution of ideal gases
The figure below shows the speed distribution according to Maxwell-Boltzmann for...