Specific heat of solidification is the heat energy to be released for solidification of a liquid per kilogram of the substance!
Melting and solidification
In the article on specific latent heat of fusion, it was explained in detail that when a solid is to be melted, energy is required to break the bonds so that the substance changes to the liquid phase. This energy is added to the substance as heat and is called heat of fusion. In the case of pure substances, the temperature remains constant during melting.
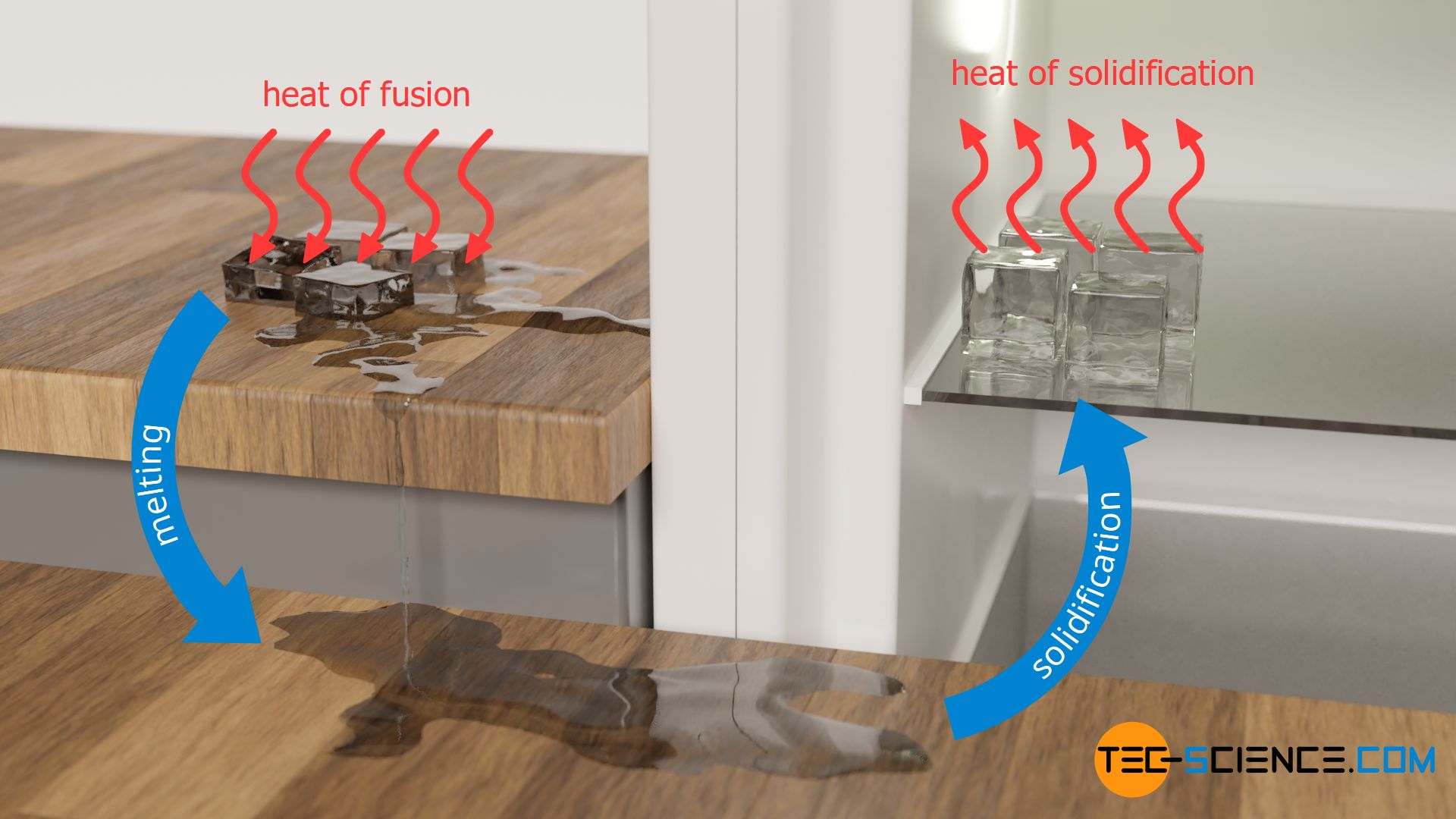
If the liquid substance is to be solidified, then the heat previously added must be released so that the molecules can bind to each other again. In this case, the heat energy to be given off during the solidification of the liquid is called heat of solidification. Even while the heat of solidification is being released, the temperature remains constant. It only drops when the liquid substance has completely solidified. The temperature at which a substance solidifies corresponds to the melting point of the substance (also called solidification point).
For a more detailed explanation of the molecular processes that occur during solidification and the reason why the temperature remains constant, see article Why does the temperature remain constant during a change of state (phase transition)?
Specific heat of fusion and solidification
Due to the conservation of energy, the amount of heat absorbed by a substance to melt (heat of fusion) is equal to the amount of heat released by the substance during solidification (heat of solidification). For this reason, the specific heat of solidification qs, as the ratio of the heat of solidification Qs and the solidifying mass ms, is as great as the specific heat of fusion qf.
\begin{align}
&\boxed{q_\text{s} = \frac{Q_\text{s}}{m_\text{s}}}~~~\text{where}~~~\boxed{q_\text{s} = q_\text{f}} \\[5px]
\end{align}
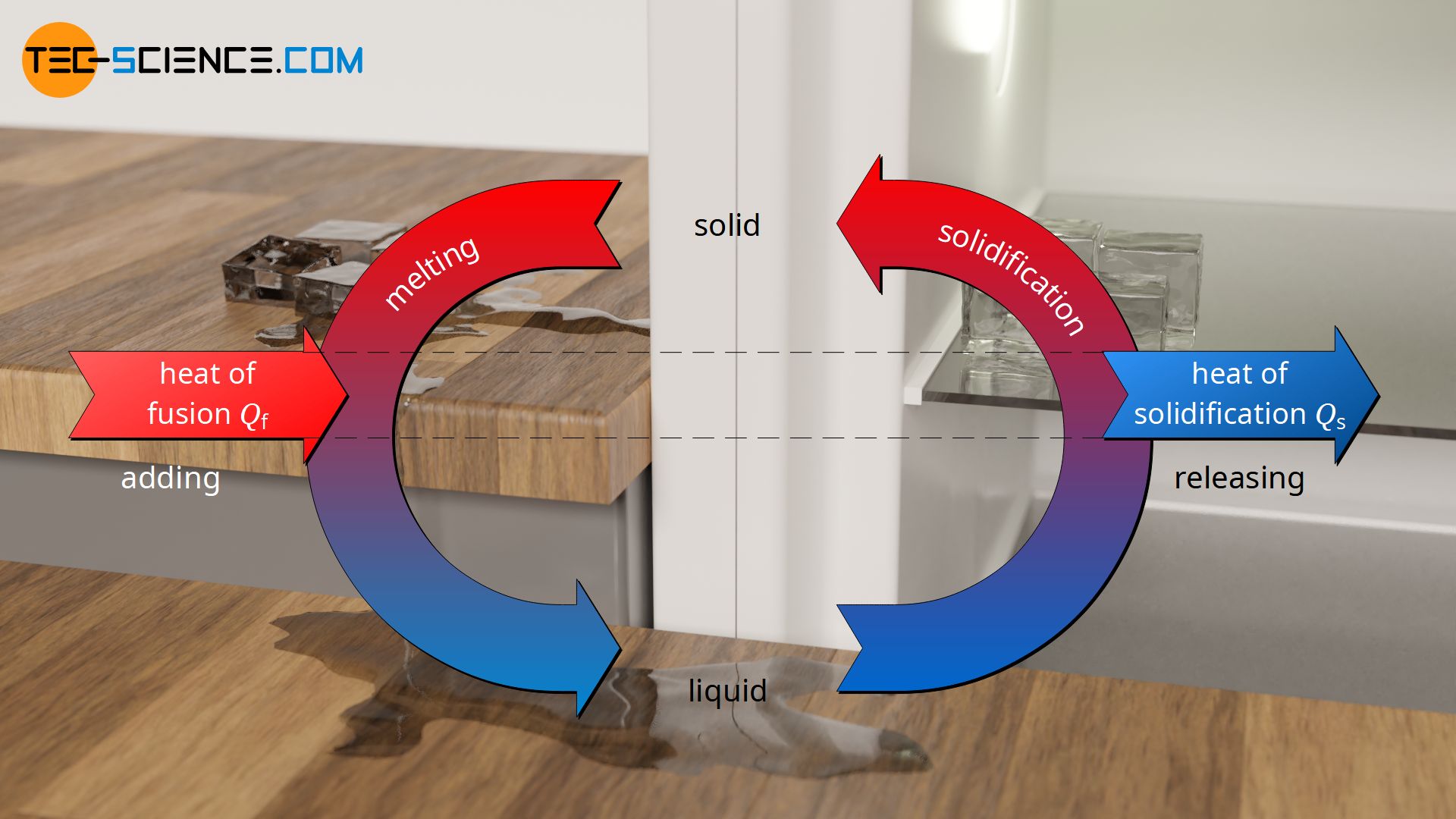
In the article Specific latent heat of fusion, the specific heats of fusion or solidification are listed for selected substances. Using these values, the heat of solidification Qs to be released for a given mass ms to be solidified can be determined with the following formula:
\begin{align}
&\boxed{Q_\text{s} = m_\text{s} \cdot q_\text{s}}\\[5px]
\end{align}
In the case of solidification, again one speaks of latent heat, since the heat to be released during solidification is not directly evidenced by a change in temperature (from the Latin word “latere”, which means “to be hidden” or “not to appear directly”).
In the case of water, the latent heat to be released during solidification is 334 kJ per kilogram, which is about the same as the amount of heat that would be needed to bring the water to a boil, starting at room temperature. This is therefore a very large amount of heat that has to be released or that is given off by the liquid water during solidification. This explains, for example, why sprinkling plants with water at temperatures below freezing point is used as frost protection.






