The specific heat capacity of water depends on the temperature and is strongly dependent on the state of matter.
The specific heat capacity is not a material constant for a substance, but depends on the temperature and above all on the state of matter. The dependence of the specific heat capacity on the state of matter and on the temperature is to be shown using the example of water.
The minimum value of the specific heat capacity of liquid water with about 4.18 kJ/(kg⋅K) will be found at a temperature of 40 °C and the maximum value with about 4.22 kJ/(kg⋅K) at a temperature of 0 °C. If an average value of 4.2 kJ/(kg⋅K) is assumed for liquid water, the deviation from the maximum or minimum value is less than 1 %! Thus, for many practical cases the specific heat capacity of liquid water can actually be assumed to be constant.
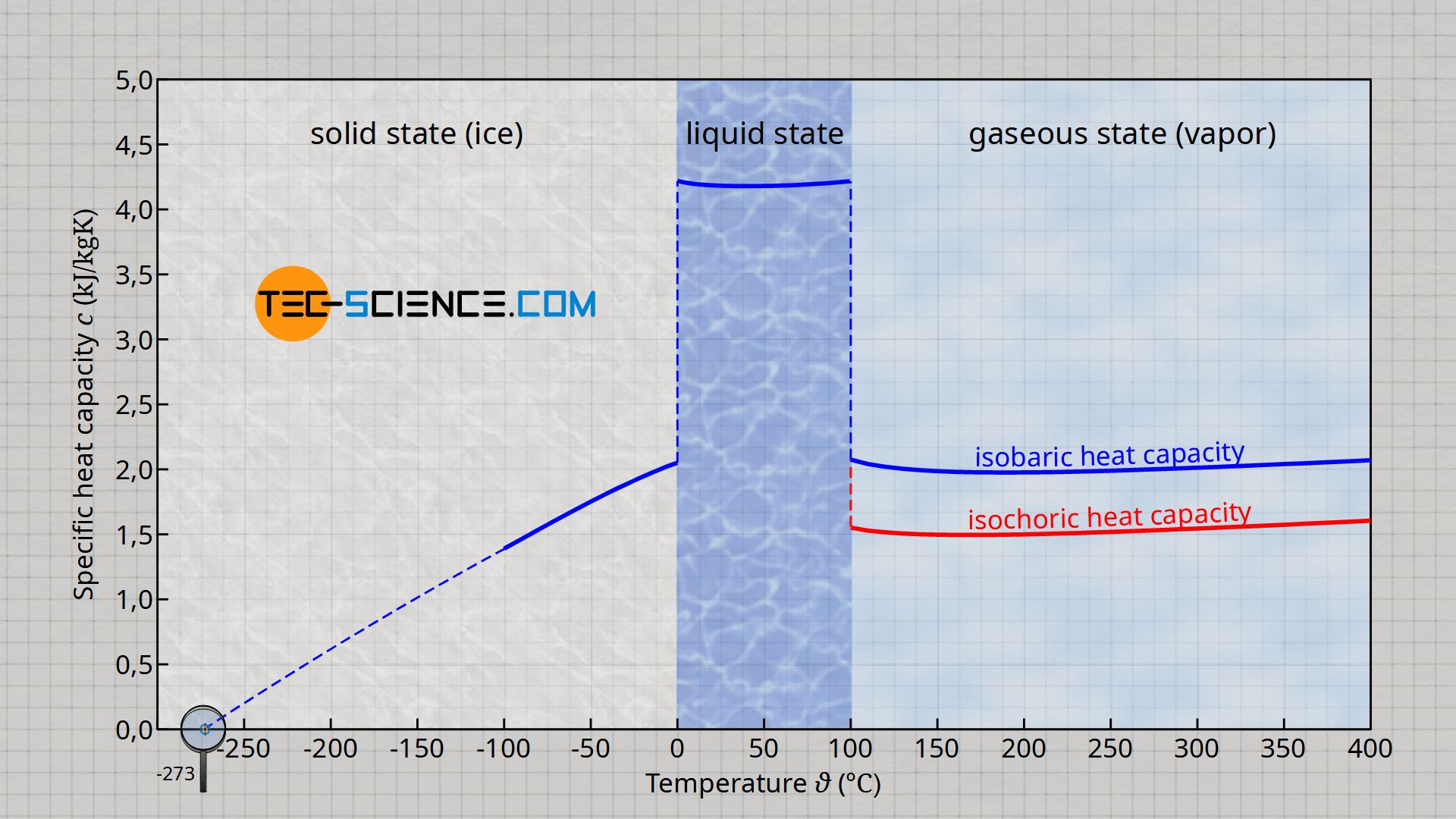
The diagram above shows the specific heat capacity of water as a function of temperature for the different states of matter. It is clear that the specific heat capacity changes considerably when there is a phase transition. In the gaseous state, the specific (isobaric) heat capacity is only about half as large as in the liquid state. But it is also true for the gaseous state that the specific heat capacity can be regarded as almost independent of the temperature.
The situation is different for solid water (ice). In this case, the specific heat capacity changes very strongly with the temperature. For low temperatures it decreases more and more and is even zero at absolute zero! Such a behavior does not only show water but every substance.
The specific heat capacity of substances is zero at absolute zero!
In the gaseous state of water (as with all other compressible substances), a distinction must be made as to whether heat transfer takes place at constant pressure or at constant volume. Accordingly, a distinction is made between the isobaric specific heat capacity cp and the isochoric specific heat capacity cv. The isobaric specific heat capacity is always larger than the isochoric specific heat capacity. More information can be found in the article Specific heat capacity of gases (at constant volume or pressure).






