Find out in this article why metal feels colder than wood of the same temperature, while at higher temperatures the metal suddenly feels warmer than wood.
The property of an object to be hot or cold
If you touch a metallic object at relatively low temperatures, it feels much colder than an object made of wood at the same temperature. On the other hand, at higher temperatures metal suddenly feels warmer than wood of the same temperature. For this reason, handles for wood-burning stoves are made of wood that can be touched by hand despite high temperatures compared to some metal handles.


The misunderstood concept of heat
This paradox lies in the often misunderstood concept of heat. In everyday life we misunderstand the term heat as a property of an object to be warm or cold. And mistakenly we attribute this property to temperature. The reason for this is our deceiving everyday experience, since in most cases a warm feeling is indeed often connected with a high temperature and a cold feeling with a low temperature.
However, even a simple experiment shows that this relationship between temperature and the feeling of warm or cold is not always true. The experiment is carried out by pouring water into a pot and waiting until the water has reached room temperature of about 25 °C. Then you dip a hand into the water. The water will feel relatively cold. So we would claim that a temperature of 25 °C feels cold in case of water (at least most of us would not want to take a bath with a water temperature of 25 °C).

Now we repeat the experiment. This time, however, we dip our hand in water filled with ice cubes with a temperature of 0°C for about one minute before carring out the experiment. Straight after that we put our hand into the pot with water of 25°C. We would now come to the conclusion that the water suddenly feels very warm, although the temperature is also 25°C (or even a bit lower, since the water is still cooling down by the cold hand!) We would now conclude that a temperature of 25°C feels warm in the case of water.

Although the water temperature has not changed, we feel the water as cold or warm depending on what we have done with our hands. This makes it evident that we humans obviously have no direct sense organ for temperatures! Therefore, we must not equate temperatures in general with the feeling of cold and warm.
Heat flows as perception for warm and cold
In this context, the question arises how we humans then feel warm and cold if not by temperature. In fact, our skin does not react directly to temperatures, but to heat flows! And these heat flows in turn depend on the temperature difference between skin and object we touch. Temperature differences ultimately drive the flow of heat. Heat energy always flows from spots of higher temperature to spots of lower temperature (see also the article Rate of heat flow: Definition and direction). This is also the reason why a hot cup of coffee cools down over time and does not heat up further by itself, as heat flows from the hot coffee to the cooler surrounding.

If the human skin is exposed to a large heat flow (i.e. when a lot of heat energy is transferred in a short time), the brain perceives this as a warm feeling. Accordingly, our brain perceives a low heat flow to our skin as less warm. In these cases the temperature of the objects we touch is higher than the temperature of our skin.
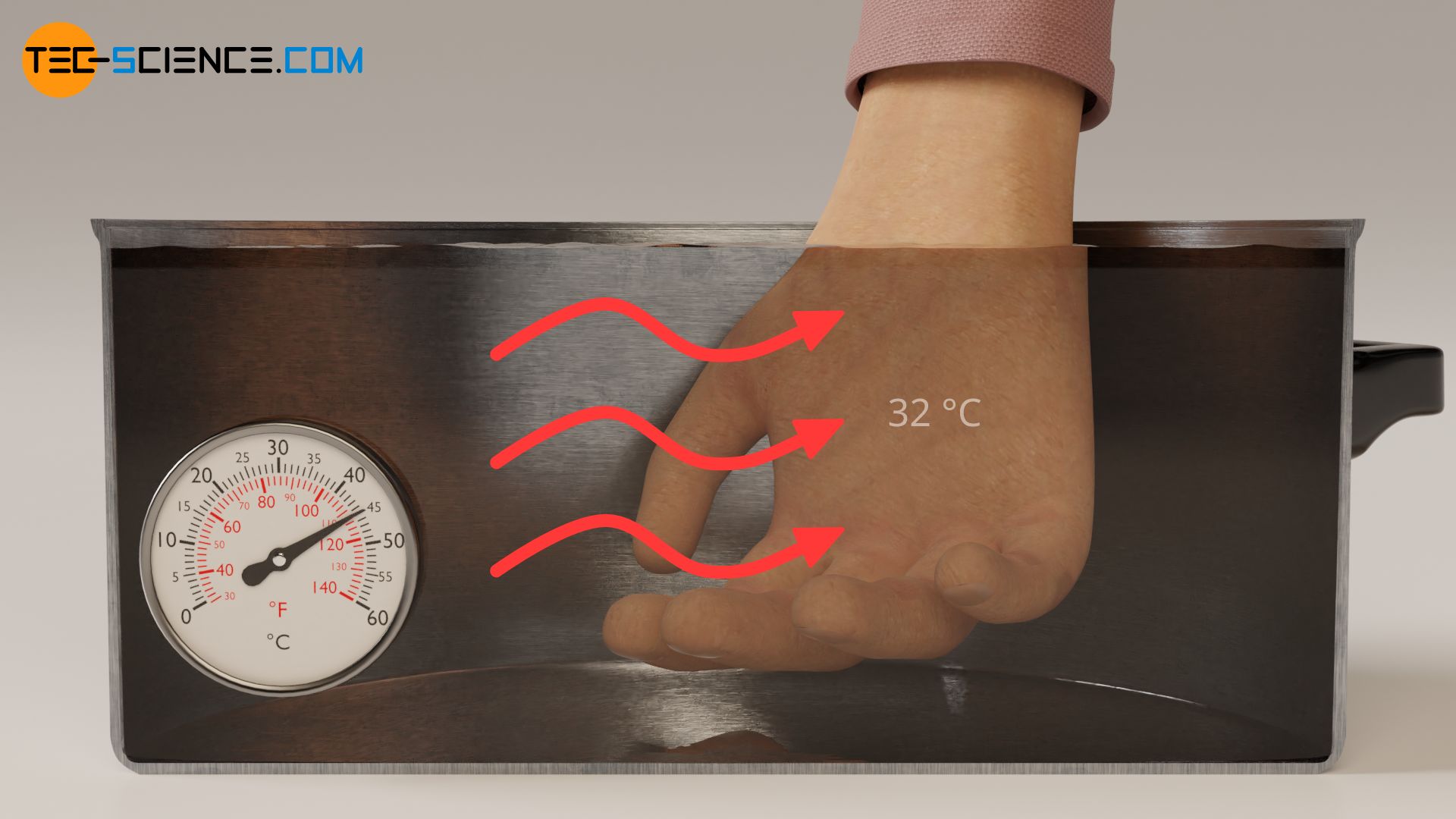
Conversely, we feel objects as cold when the heat flow is directed away from our skin. If a lot of heat flows away from our skin in a short time (large outgoing heat flow), we perceive this as very cold. If the outgoing heat flow is relatively small, the object feels less cold. In these cases the temperature of the objects we touch is lower than the temperature of our skin.
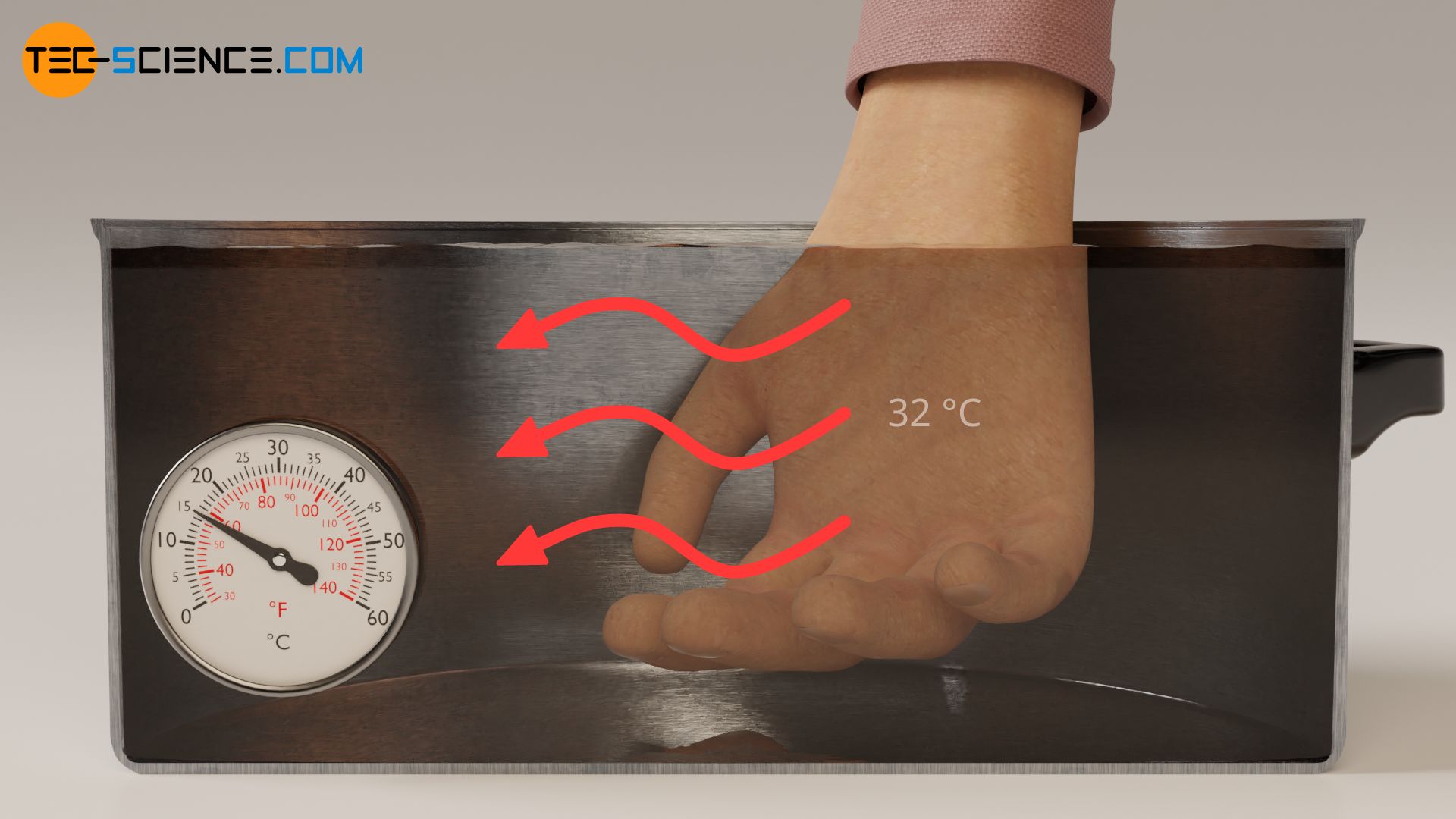
A heat flow directed away from our skin is perceived as cold and a heat flow directed towards our skin is perceived as warm. The rate of heat flow and thus the intensity of the sensation of how cold or warm an object is depends on the temperature difference between skin and object!
If no heat flows from an object to our skin or from our skin to an object, the object feels neither warm nor cold. In this case the object has the same temperature as the surface of our skin (approx. 32 °C). So we see that only temperature differences determine the rate of heat flow and that the direction of the heat flow in particular determines the feeling of warm and cold and not the temperature of an object itself.
Why does metal feel warmer/colder than wood?
Different materials such as metal and wood differ in their property to conduct heat at a given temperature difference to a greater or lesser extent. We express this property among other things by the thermal conductivity. Metals have a significantly higher thermal conductivity than wood. This means that metals can transfer more heat per unit time than wood at the same temperature difference (see also Thermal conductivity (Fourier’s law)).
So if we touch a metal bar at a temperature of 40 °C, the metal is able to transfer more heat than wood in the same time due to its higher thermal conductivity. The rate of heat flow is greater and therefore the metal feels much warmer than wood.
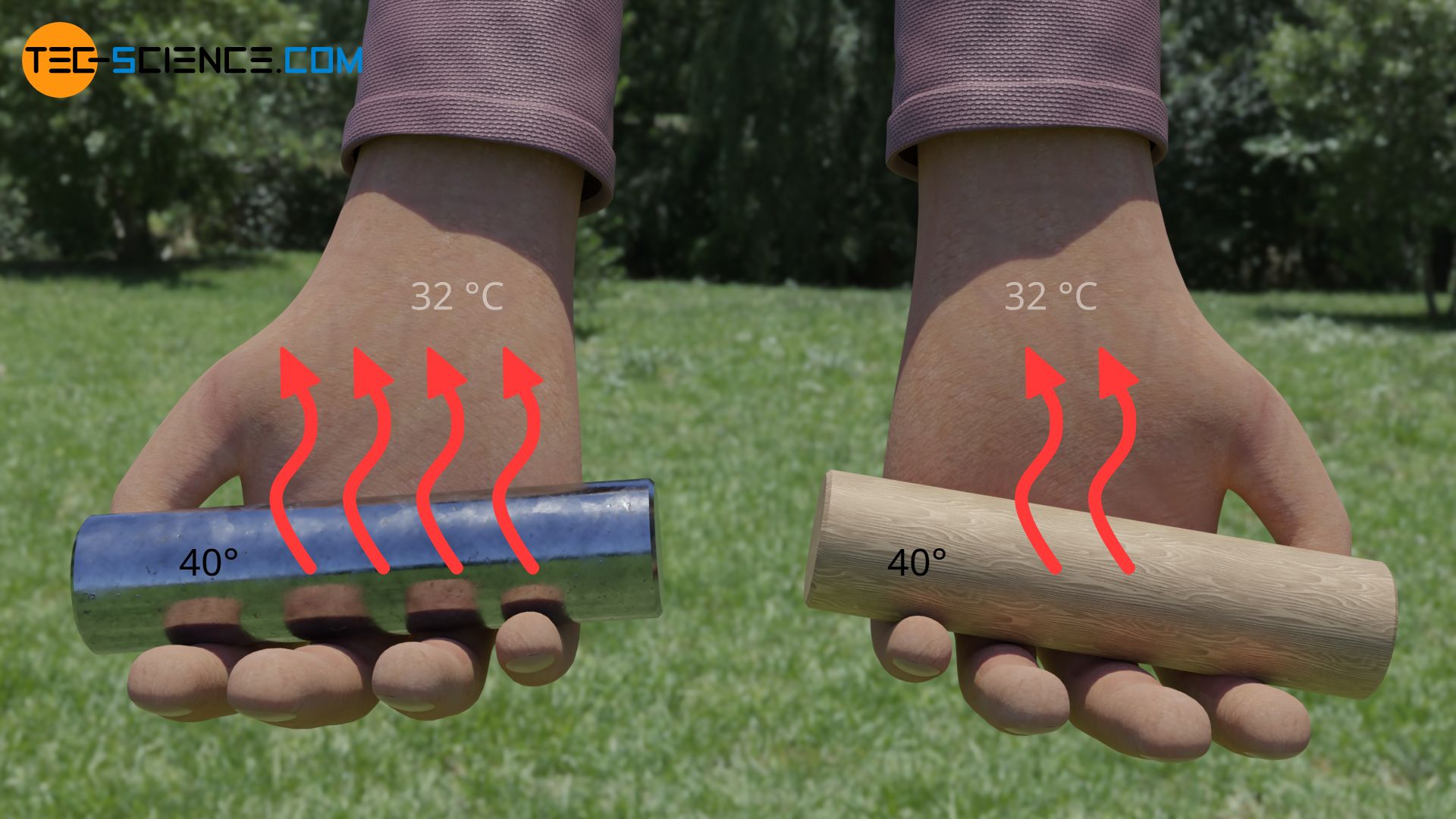
Conversely, the high thermal conductivity of metals also means that more heat can be transferred away from our skin at cold temperatures than is the case with wood. The rate of heat flow away from our skin is greater with metals and the feeling of cold is more intense. This is why metals feel colder than wood at lower temperatures.
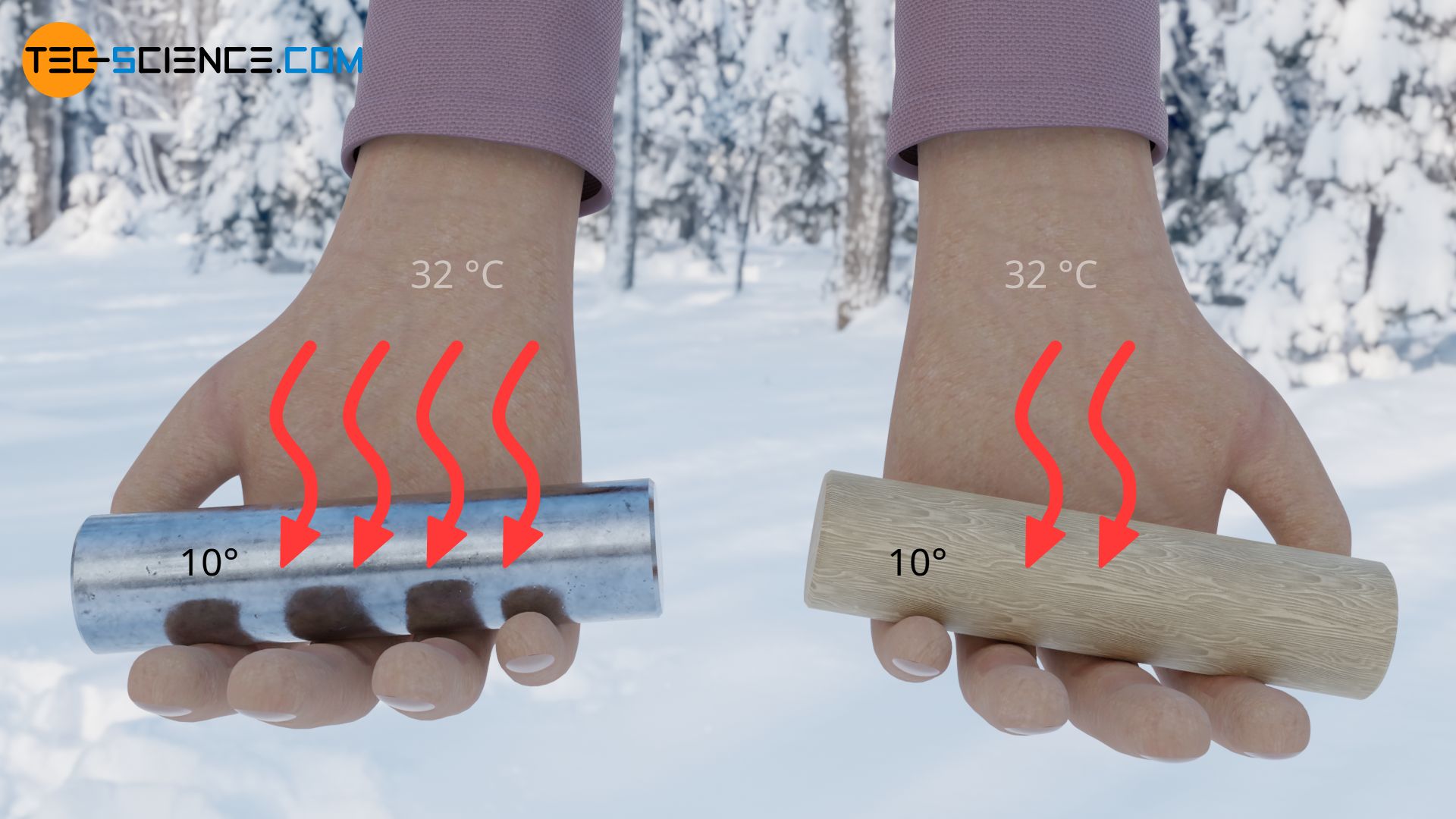
In fact, not only the thermal conductivity of the material is important for the rate of heat flow, but also its density and specific heat capacity (see also thermal diffusivity).
Why does cold water suddenly feel warm?
With the same reasoning as above, it can also be explained why cold water suddenly feels warm if you have held your hand in ice water for some time before. In the normal state, our skin has a surface temperature of around 32 °C (see thermal image below). If we dip our hand in this state into the pot of water at 25 °C, heat flows from our skin (higher temperature) to the water (lower temperature). Due to the heat flow away from our skin the water feels cold.
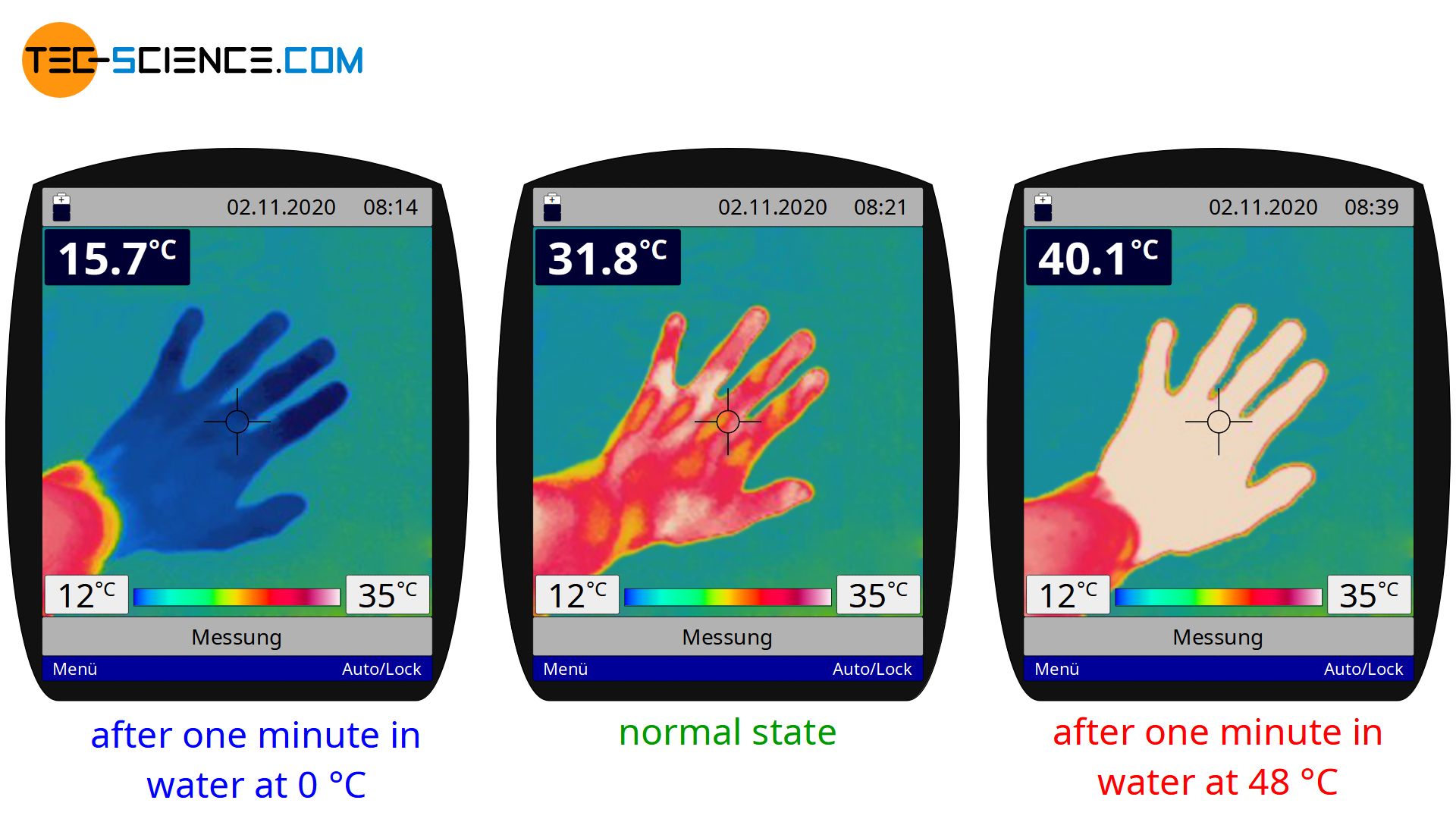
However, if we dip the hand in ice water beforehand, the surface of the skin and our sensory cells cool down to about 16 °C. If we place the hand in this state in the pot of water at 25 °C, the direction of heat flow is now reversed. Now heat flows from the water (higher temperature) to our skin (lower temperature). Due to the heat flow directed to our skin, the water now feels warm.
We can intensify the feeling of warm and cold by further increasing the temperature difference between skin and water before the experiment. If, for example, we leave the hand in ice water for a longer time, it will cool down more strongly. The temperature difference is then later greater and so is the sensation of warmth. The intensity of the feeling of cold water can be increased by placing the hand in even hotter water before the experiment. The skin temperature increases and with it the temperature difference between our skin and the water of 25 °C (see thermal image above). The cold feeling is then intensified.
In this context the often heard statement at a swimming lake has to be interpreted: “once you are in the water, it doesn’t feel so cold anymore“. This is because the skin cools down after a short time in cold water. The lower skin temperature thus reduces the temperature difference to water. From now on this results in a reduced heat flow and the water no longer feels so cold.






