Heat capacity of objects
Heat capacity is the amount of heat required to raise the temperature of an object by 1 Kelvin (1 °C). Learn more about it in this article.
Specific heat capacity of substances
The...
Important remarks on the specific heat capacity
https://www.youtube.com/watch?v=0JZOK2hcQok
Definition of the specific heat capacity
The specific heat capacity c describes the relationship between a transfer of heat Q and the associated temperature change ΔT of a substance of mass m:
begin{align}label{q}&...
The process quantities: Heat and work
Work and heat are process quantities that describe the process of a supply of energy ("energy in transit")! Learn more about it in this article.
Work and heat transferred to a substance
Energy...
Vineyard Frost Protection (sprinkling with water)
With sprinklers for frost protection, the crop stays protected from low temperatures by the heat of solidification released when the water freezes.
https://www.youtube.com/watch?v=bh43jUpB_F4
If growing fruits are exposed to sub-zero temperatures (frost) on...
Dissipative thermodynamic processes in adiabatic systems
In this article, learn more about dissipative thermodynamic processes using the polytropic equations.
Work performed in adiabatic systems
Many thermodynamic processes take place within very short times, such as the expansion of the...
Heating and cooling of several objects
Learn more about calculating the final temperature of several objects with different temperatures in this article.
Heating of several objects
In practice, when heating or cooling objects, one usually has to deal with...
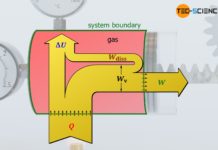
Dissipation of energy in closed systems
Learn in this article why, in thermodynamic processes with dissipation of energy, the pressure-volume work of the gas does not correspond to the work done by the system.
Pressure-volume work (displacement work)
In...