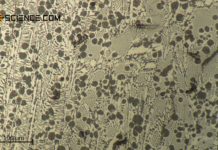
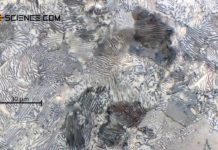
In this article, a summary is given about the phase transformations during solidification and cooling of steel.
Introduction
In the article Phase transformations in the solidified state the microstructural changes of steels during cooling were explained in great detail. Since these transformations are very complex, a brief overview of the microstructural transformations is given in this summarizing article. More detailed information can be found in the article Phase transformations of steels in solidified state (metastable system).
Solidification
The actual solidification process in steels takes place independently of the carbon content as in a solid solution alloy. This is shown in the phase diagram as a typical lenticular region between the liquidus and the solidus line. The carbon is completely soluble in the face-centered cubic γ-iron lattice structure immediately after solidification. This solid solution of face-centered cubic iron and embedded carbon therein is called austenite.
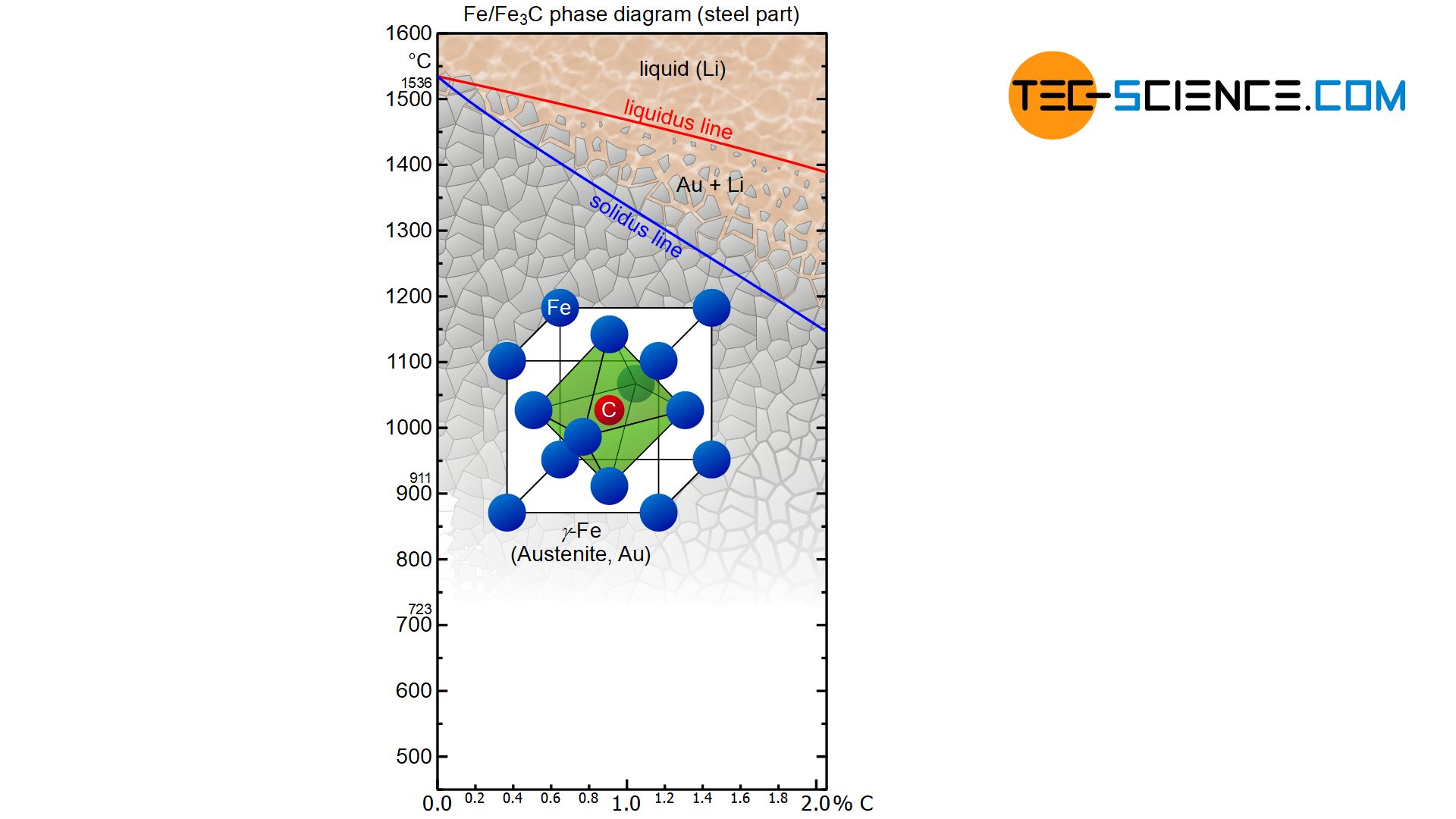
In the solidified state, the iron-carbon phase diagram shows the typical horizontal “K” of a crystal mixture, in which the respective components are insoluble in one another. Note that carbon in iron is actually almost insoluble at room temperature. The phase transformations that the steel undergoes in the further cooling process can therefore be considered in analogy to a crystal mixture alloy. However, the phase transformations take place in an already solidified state!
Phase transformations in solidified state
Hypereutectoid steels
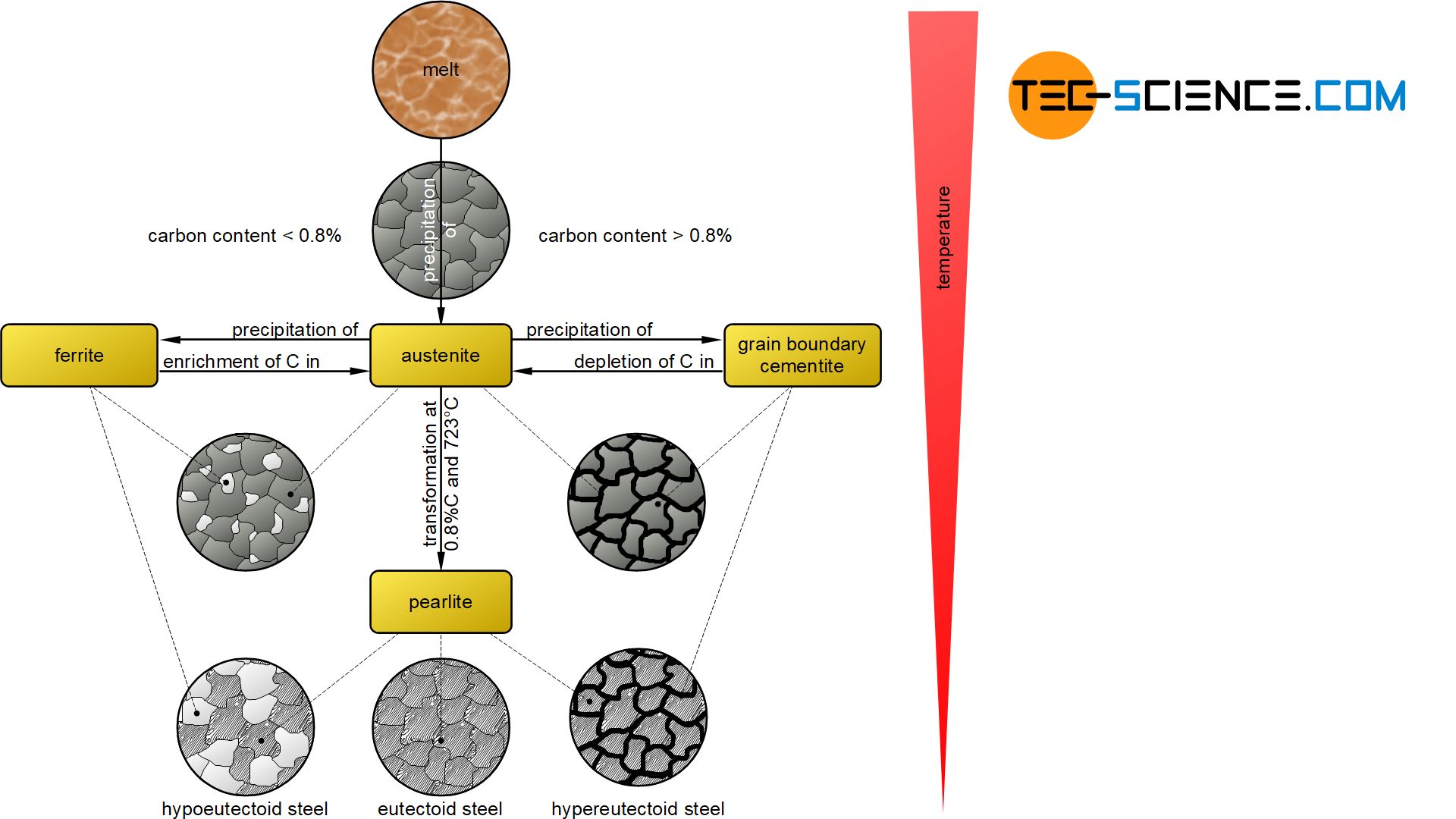
In hypereutectoid steels with a carbon content of more than 0.8 %, carbon in the form of cementite precipitates at the grain boundaries when the solubility limit is reached (grain boundary cementite). This leads to a depletion of carbon in the remaining austenite. Depletion finally progresses until the retained austenite reaches the eutectoid composition of 0.8 % carbon at 723°C.
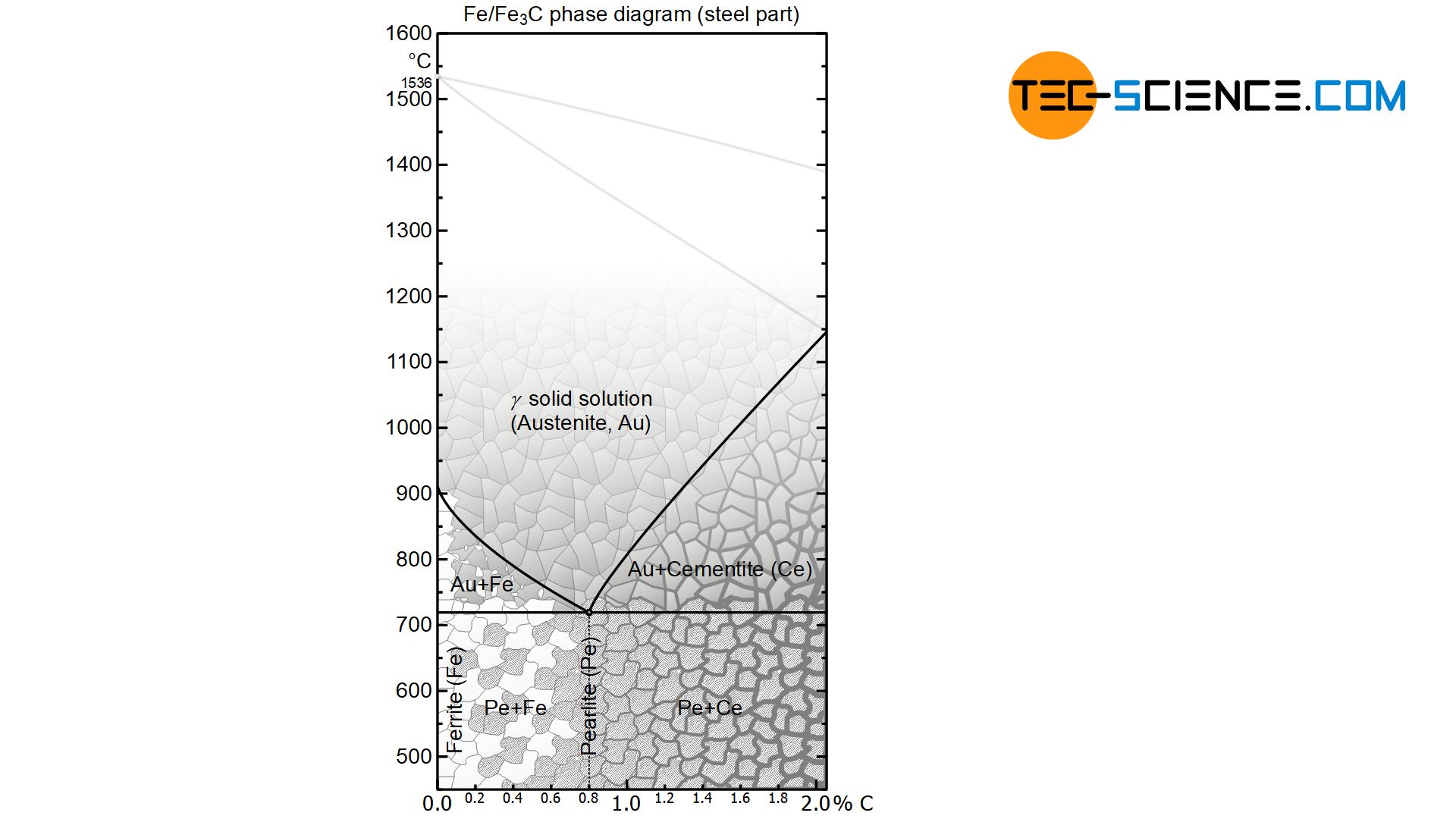
Now, at a constant temperature of 723 °C, the face-centered cubic austenite begins to convert completely into the body-centered cubic ferrite structure. However, the carbon can no longer be dissolved in the ferrite lattice. Therefore the carbon precipitates directly out of the ferrite in the form of cementite lamellae. This eutectoid phase mixture of ferrite grains with the cementite lamellae embedded therein is also known as pearlite.
The microstructure of a hypereutectoid steel at room temperature consists of the previously precipitated grain boundary cementite and the pearlite formed.
Hypoeutectoid steels
For hypoeutectoid steels with a carbon content of less than 0.8 %, ferrite is precipitated from the austenite lattice when the temperature falls below the γ-α-transformation line, as the face-centered cubic austenite begins to transform into the body-centered cubic ferrite.
The carbon that can no longer be dissolved in the ferrite lattice formed diffuses into the surrounding austenite lattice, as it can still absorb carbon (under-saturated state). This leads to an accumulation of carbon in the remaining austenite. The enrichment finally progresses until the retained austenite has reached the eutectoid composition of 0.8 % carbon at 723 °C.
Now the residual austenite again transforms into pearlite. Note, that the formation of pearlite is always identical regardless of the carbon content of the steel.
At room temperature, the microstructure of a hypoeutectoid steel thus consists of the previously separated ferrite grains and the pearlite formed.
Eutectoid steels
In a eutectoid steel with exactly 0.8 % carbon, the austenite has the eutectoid composition from the very beginning. Thus, pearlite will form directly from the austenite without precipitation processes.
The microstructure of a eutectoid steel consists only of pearlite grains at room temperature.
Note that the microstructure of the steel is always composed of the two phases ferrite and cementite, regardless of whether it is a hypoeutectoid (hypopearlitic) steel or a hypereutectoid (hyperpearlitic) steel. This is precisely the characteristic of the metastable system.
The determination of the microstructure fractions of pearlite and ferrite is explained in the article Determination of microstructure and phase fractions.