Depending on the carbon content, further phase transformations take place in the steel in the solidified state. The cooled microstructure consists of pearlite and ferrite.
Introduction
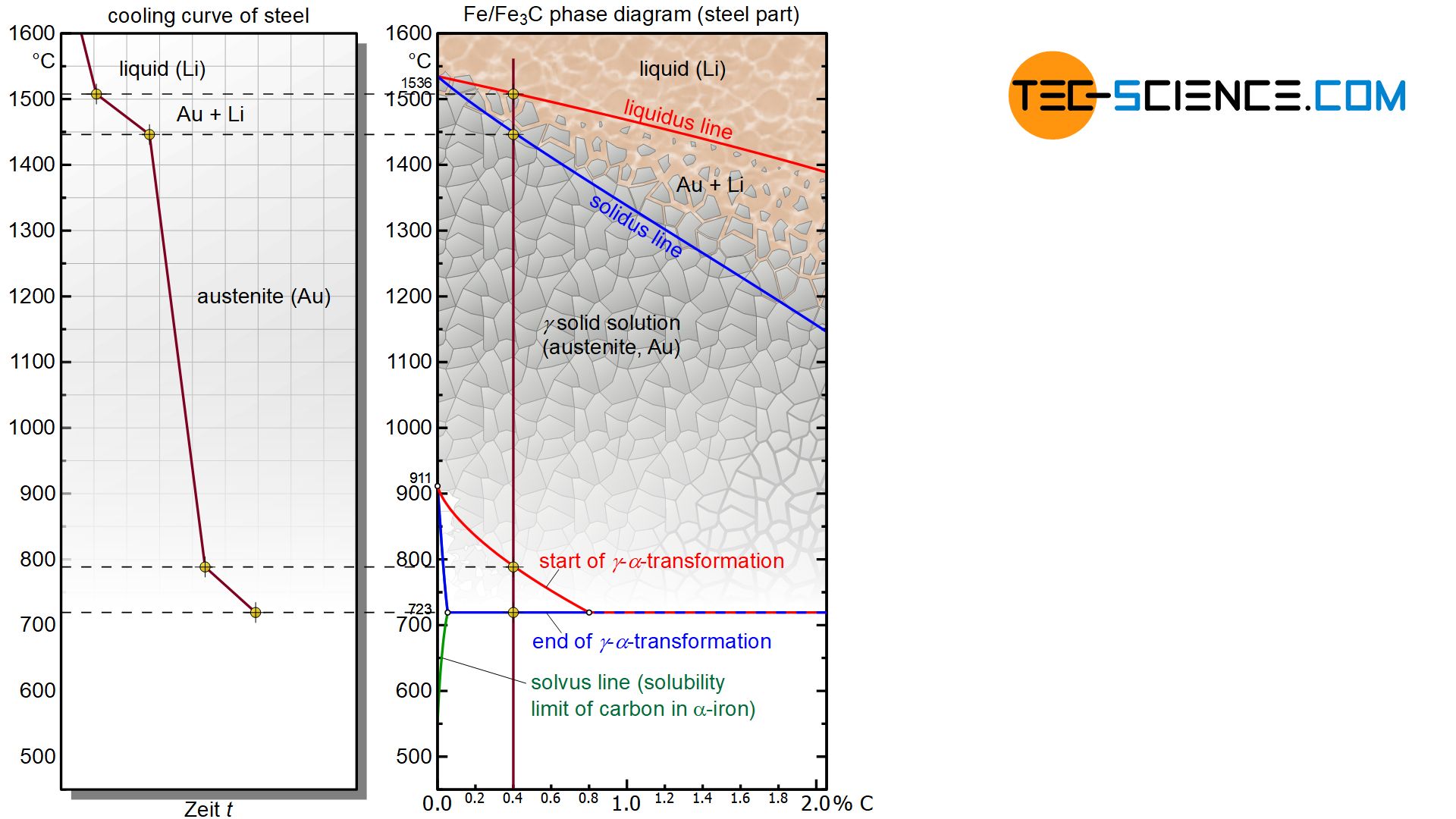
As explained in the article Microstructure formation of steels during solidification, carbon affects the temperature of the γ-α-transformation. As the carbon concentration increases, the start of the transformation decreases from 911 °C for pure iron to lower temperatures and finally remains constant at a value of 723 °C from a carbon concentration of 0.8 %.
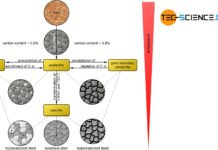
Accordingly, three different types of steel can be distinguished, each of which undergo typical microstructural changes during cooling:
- eutectoid steels with a carbon content of exactly 0.8%!
- hypereutectoid steels with a carbon content greater than 0.8%!
- hypoeutectoid steels with a carbon content of less than 0.8%!
The different phase transformations during cooling from the austenitic state are described in more detail in the following sections.
Eutectoid phase transformation
The changes in the microstructure during the γ-α-transformation are explained in more detail below using the so-called eutectoid steel C80 with a carbon content of 0.8%.
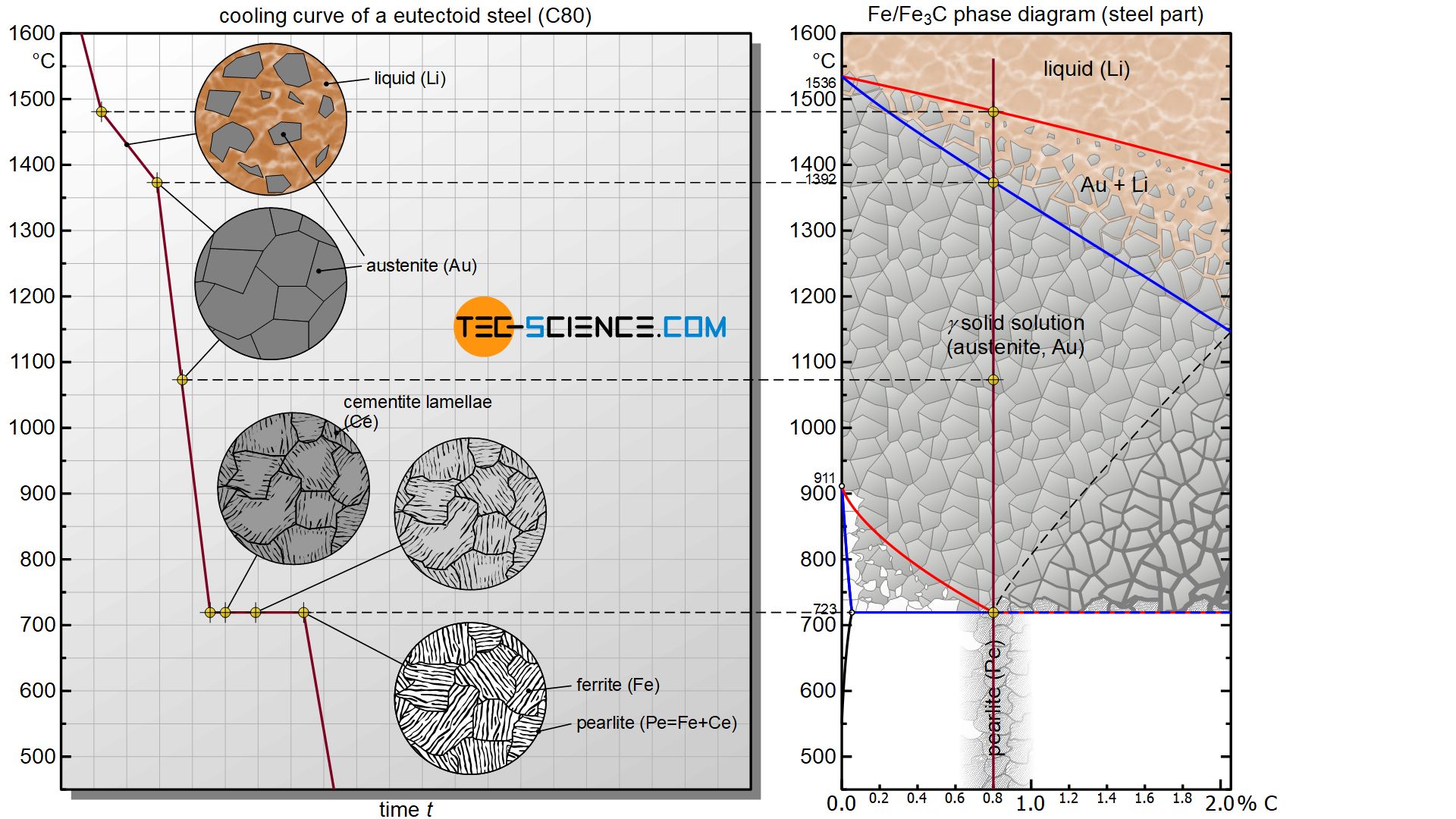
After the steel has solidified in a temperature range and the solid solution austenite structure has formed, the steel is finally subjected to the γ-α-transformation at a temperature of 723 °C. Now the face-centered cubic lattice structure of the austenite begins to transform into the body-centered cubic lattice structure of ferrite at constant temperature (thermal arrest).
Since the centre of the cube is already occupied by an iron atom in the ferrite grid, the carbon atom can no longer be dissolved in it. During this lattice transformation, the carbon is precipitated in the metastable form of iron carbide Fe3C (cementite). Due to the relatively low temperature of 723 °C, the precipitated atoms are slow and can therfore not travel long distances. They are therefore precipitated directly from the lattice structure and are deposited next to each other in a lamellar structure.
Once austenite has completely transformed into ferrite, the carbon is (almost) completely separated from the iron lattice structure. The former austenite grains have now become ferrite grains with embedded cementite lamellae. This lamellar two-phase mixture of ferrite and cementite is also known as pearlite due to the pearlescent sheen under the microscope.
Pearlite is the eutectoid phase mixture formed at 723 °C, consisting of ferrite and embedded cementite lamellae!
Note that during the γ-α-transformation the outer shape of the grains changes too! While austenite is more polyhedral and thus has an angular grain structure, the pearlite grains are rather roundish. The polyhedral form of austenite is caused by the increased formation of so-called twin grain boundaries. The change of the grain shape during the γ-α-transformation is used e.g. during so-called normalizing in order to eliminate unevenly large grains in a microstructure and thus achieve a homogeneous grain refinement.
Due to its lamellar structure, the pearlite structure is very similar to the eutectic structure of an alloy with components insoluble in one another. The only difference that an eutectic forms from the liquid state while the pearlite structure forms from the already solidified state. In contrast to this, this phase mixture is therefore not called eutectic but eutectoid.
In the present case, the steel with a carbon content of 0.8 % thus has a purely eutectoid structure. Such a steel is therefore also called eutectoid steel or pearlitic steel.
Eutectoid steels have a purely pearlitic structure with 0.8 % carbon at room temperature (ferrite grains with embedded cementite lamellae)!
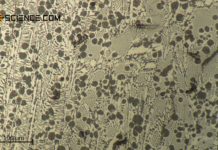
The micrograph below shows a pearlitic steel with 0.8 % carbon. The strip-shaped embedded cementite lamellae (dark stripes) in the ferrite grains (light areas in between) can be seen.
However, a purely eutectoid pearlite structure is only present if the steel has a carbon content of exactly 0.8 %. The effect a higher carbon concentration has on the structure of the steel is examined in more detail in the following section.
Hypereutectoid phase transformation
Limited solubility of carbon in austenite
Even in the case of hypereutectoid steels with a carbon content of more than 0.8 %, the microstructure is initially present as a pure solid solution structure immediately after solidification (austenite).
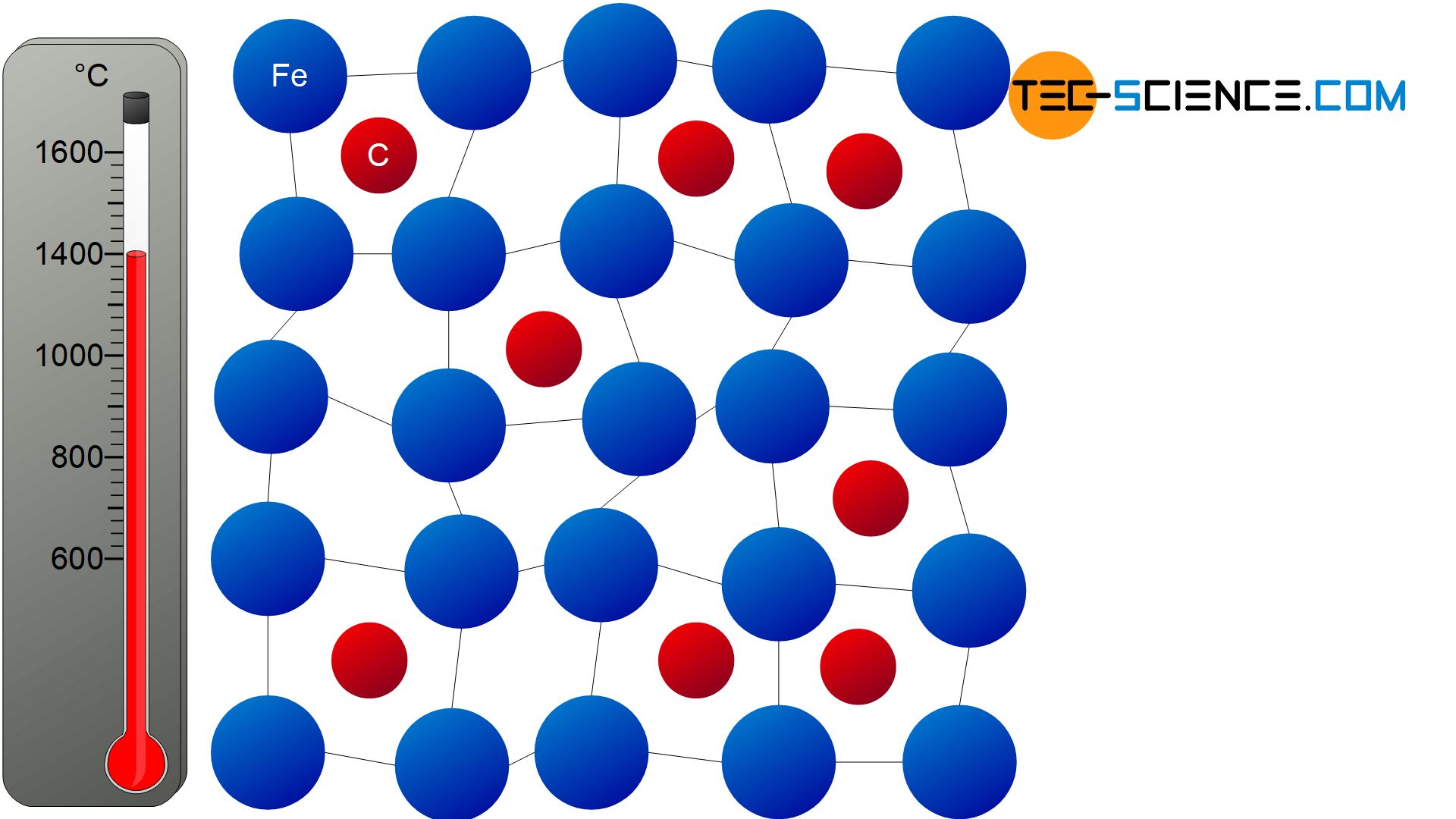
Although carbon is relatively well soluble in this austenite structure, its solubility is not unlimited. The carbon atoms are relatively large compared to the gaps in the center of the face-centered cubic unit cells. If the iron atoms are assumed to be touching spheres, there is a gap in the middle of the cube into which a sphere with a maximum of 0.4 times the diameter of the iron atoms fits. However, the carbon atoms have about 0.6 times the diameter.
This means that the carbon atoms are actually too large to fit easily into the middle of the unit cells. As a result, lattice distortions occur in the vicinity of the embedded carbon atoms. Finally, no further carbon atom can be incorporated within the distorted lattice area because the lattice distortions are too strong. Only at certain intervals can further carbon atoms be stored again. The solubility of the colon atoms in γ-iron is thus limited.
The maximum number of carbon atoms that can be dissolved in the austenite lattice depends to a large extent on the temperature. For example, a lower temperature also means a reduced lattice vibration and thus the space within the unit cells also becomes smaller with decreasing temperature. As a result, fewer carbon atoms can be dissolved in the austenite microstructure. Consequently, the solubility of carbon atoms decreases with decreasing temperature! Conversely, a higher temperature means a higher solubility.
The solubility of carbon in austenite decreases with decreasing temperature!
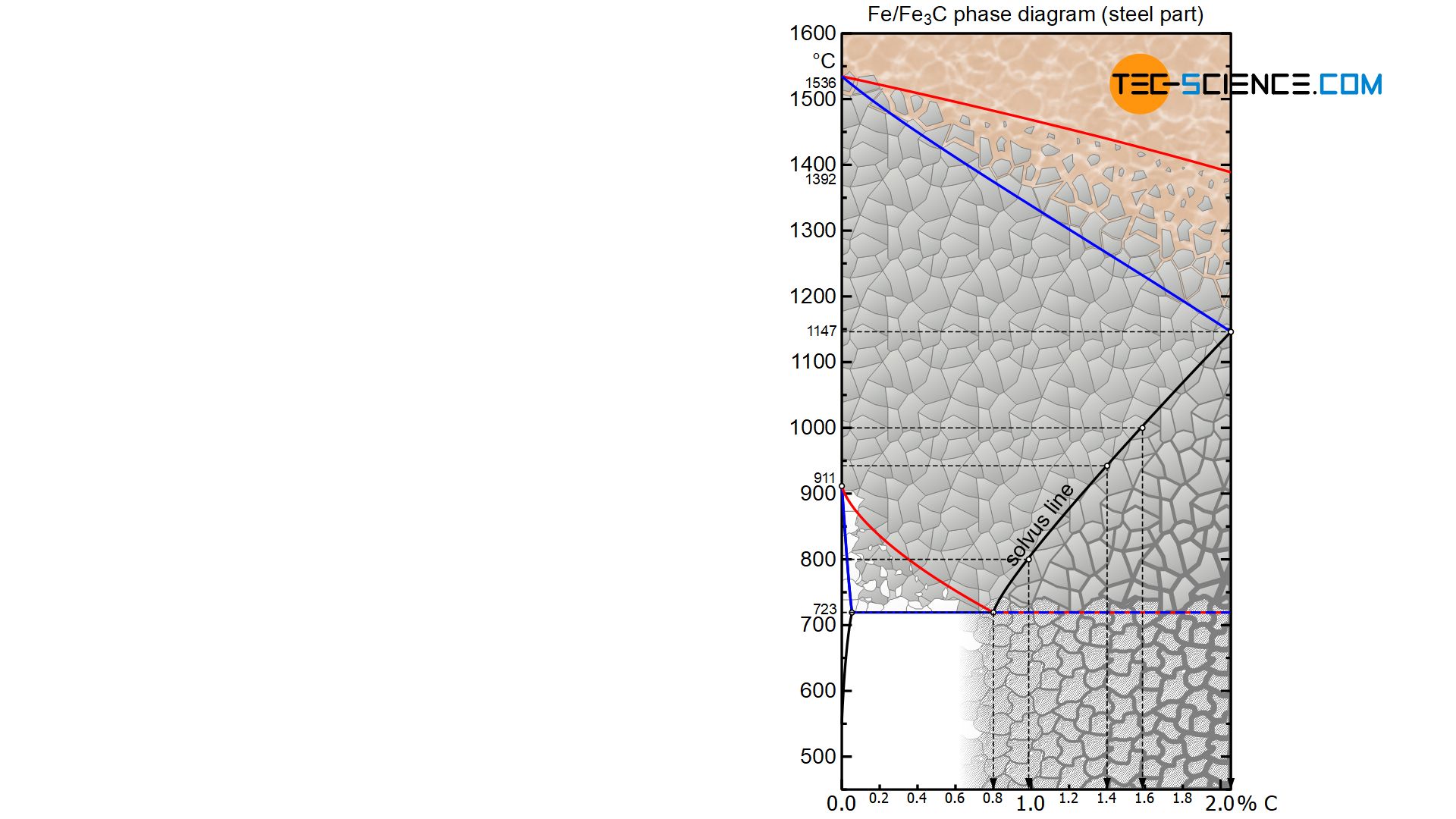
The maximum possible solubility of carbon in austenite is shown at a temperature of 1147 °C with 2.06 %. Every 2nd to 3rd elementary cell (unit cell) is occupied with a carbon atom. As the temperature decreases, the solubility decreases continuously and immediately bevore disintegration of the austenite into the body-centered cubic lattice structure at 723 °C the solubility is only 0.8 % at most. The carbon atoms only find a place in every 6th to 7th unit cell.
Using the solubility limit drawn in the iron-carbon diagram (called solvus line), the corresponding maximum carbon content to be dissolved can finally be determined for every other temperature in the austenite. At a temperature of 1000 °C, for example, the maximum solubility of carbon is around 1.6 %, while the solubility at 940 °C is only around 1.4 % and has even fallen to around 1.0 % at 800 °C.
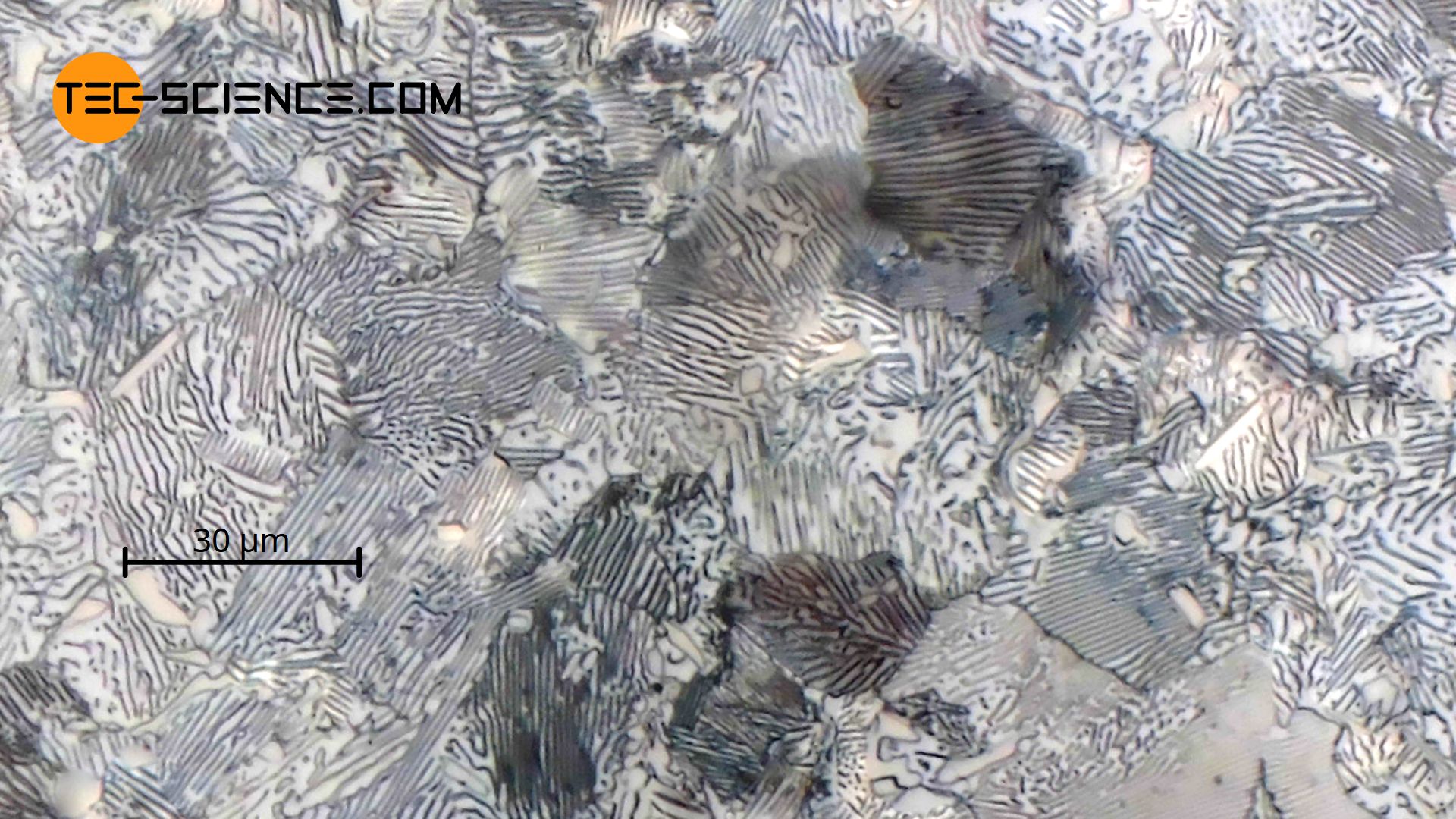
Microstructural change of a hypereutectoid steel
Due to the limited solubility, microstructural changes occur during cooling of hypereutectoid steels as soon as the solubility limit is overshot, since the steel then obviously contains more carbon than the lattice structure can actually dissolve. Using the example of a steel with 1.4 % carbon (C140), the microstructural changes that take place are described in more detail below.
At first, a hypereutectoid steel solidifies like any other steel as solid solution within a temperature range. Due to the high temperatures immediately after solidification, all carbon is initially completely soluble in the austenite microstructure.
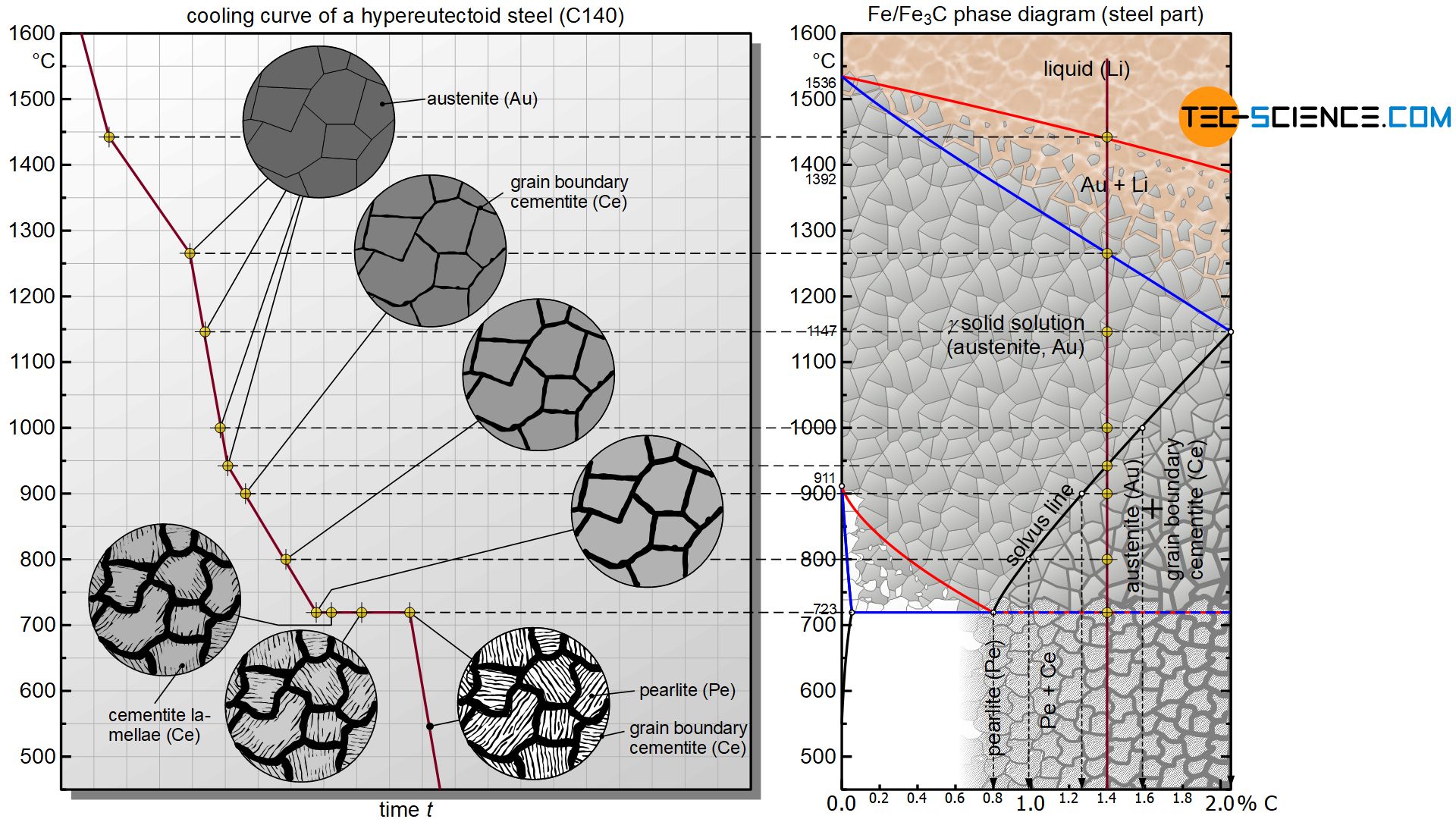
Finally, the solubility starts to decrease continuously from a temperature of 1147 °C according to the marked solvus line. At 1000 °C the maximum solubility is only about 1.6 %. However, since the considered steel has a lower carbon content of 1.4 %, all the carbon is still soluble in the austenite lattice. Since the steel could theoretically dissolve even more carbon, this state is referred to as a undersaturated state.
Finally, the maximum solubility decreases further with decreasing temperature and has dropped to 1.4 % at 940 °C. The state point is exactly at the solubility limit. At this temperature, all the carbon contained in the steel can be completely dissolved in the austenite lattice. Since the austenite lattice is completely saturated with carbon, this state also referred to as a saturated state.
If cooling continues, the carbon content of the steel is higher than the maximum solubility. This becomes clear, for example, when looking at the temperature of 800 °C. According to the solvus line, only about 1.0 % carbon can be dissolved in the austenite lattice at this temperature; however, the steel has a carbon content of 1.4 %. The microstructure must obviously change in some way when the solubility limit is overshot (phase transformation). Otherwise there would be more carbon in the austenite lattice than could actually be dissolved in it.
If the steel is in the so-called supersaturated state shortly after falling below the solvus line (i.e. more carbon is dissolved in the lattice than it can actually absorb), the “too much” carbon is precipitated from the austenite lattice. In the metastable system, this segregation of carbon takes place in the form of cementite (Fe3C).
Cementite precipitation takes place preferably at energetically favourable locations such as grain boundaries, which is why the precipitated cementite is also called grain boundary cementite. The term secondary cementite is also frequently used.
Note that the cementite is not precipitated in lamellar form in the middle of the lattice structure as in the case of pearlite formation, since cementite precipitation during pearlite formation is caused by the transformation of the lattice structure. However, if the solubility limit is exceeded, the austenite lattice structure is retained, i.e. no lattice transformation takes place. The mechanisms of cementite precipitation during pearlite formation and when the solubility limit is overshot are therefore fundamentally different!
In hypereutectoid steels, the insolvable part of carbon in the austenite lattice precipitates in the form of cementite at the grain boundaries when cooled (grain boundary cementite)!
Cementite precipitation at the grain boundaries is ultimately accompanied by a change in the energetic state. Heat is released in the grid to counteract external cooling. Therefore, the cooling rate slows down even if the solubility limit is exceeded (flattened cooling curve).
If the solubility limit is exceeded, less and less carbon can be dissolved in the austenite with further cooling. As the cooling continues, more and more cementite precipitates at the grain boundaries. This ensures that the austenite is always saturated with carbon according to its solubility. Therefore, when the solubility limit is exceeded, the carbon concentration in the austenite always corresponds to the maximum possible solubility. This of course requires that the cooling is so slow that the carbon also has time to precipitate. Only in this way a thermodynamic equilibrium state can always be achieved.
If the steel is now further cooled, the maximum solubility and thus the carbon content in the austenite decreases more and more until it finally reaches the eutectoid composition of 0.8 % carbon at 723 °C. In principle, the austenite then behaves like a eutectoid steel, which contains exactly 0.8 % carbon.
At a constant temperature of 723 °C, the austenite begins to decompose into pearlite as the face-centered cubic austenite lattice is transformed into the completely insoluble body-centered cubic structure of ferrite. The carbon is precipitated directly from the lattice structure in the form of cementite lamellae.
With precipitating of cementite, the carbon content in the austenite decreases until the eutectoid composition is reached at 723 °C and then the austenite converts to pearlite.
After this final microstructural transformation, the cooling process is finally completed and the microstructure of the hypereutectoid steel consists of pearlite grains (ferrite grains with embedded cementite lamellae) and the cementite previously precipitated at the grain boundaries.
At room temperature, hypereutectoid steels have a pearlitic basic microstructure (ferrite grains with embedded cementite lamellae) with additionally precipitated cementite at the grain boundaries!
The micrograph below shows a hypereutectoid steel with 1.0 % carbon (C100). The pearlite grains (dark) and the cementite (white) precipitated at the grain boundaries can be seen. The fine cementite lamellae in the pearlite are difficult to dissolve by light microscopy and therefore often appear monochromatic.

In addition to hypereutectoid steels with a carbon concentration of over 0.8 %, there are also steels with carbon contents below 0.8 %. They are then referred to as hypoeutectoid steels. With such steels, other microstructural transformations take place during the cooling process. These are discussed in more detail in the following section.
Hypoeutectoid phase transformation
In the following, the cooling of a hypoeutectoid steel will be considered. A steel is called hypoeutectoid if it has a carbon content of less than 0.8 %. The microstructural transformations of a hypoeutectoid steel with 0.4 % carbon are to be explained in more detail as an example.
At first, the hypoeutectoid steel solidifies again like any other steel as a pure solid solution. Carbon is initially completely soluble in the austenite structure.
Basically, a hypoeutectoid steel has too little carbon to exceed the maximum solubility limit of the carbon in the austenite. At the lowest possible temperature of 723 °C – above which austenite even exists – the (minimum) solubility for carbon in austenite is already 0.8 %. The carbon solubility is therefore always higher than the carbon content of hypoeutectoid steels.
This can be clearly seen from the phase diagram when entering the alloy as the corresponding line. In principle, all hypoeutectoid steels will never reach or even exceed the solubility limit. All carbon remains soluble in the austenite lattice of hypoeutectoid steels at any time (at least as long as austenite is exists). The austenite microstructure is permanently in an undersaturated state, as more carbon could be dissolved in it than is contained in the steel at all!
With hypoeutectoid steels, all carbon remains soluble in the austenite lattice!
The microstructural transformation of a hypoeutectoid steel is therefore not determined by the solubility limit as with hypereutectoid steels but rather by the γ-α-transformation. Although carbon in iron causes a shift in the γ-α-transformation towards lower temperatures compared to pure iron, it will still begin at a certain temperature. The onset of the lattice transformation can be seen by the red transformation line in the iron-carbon phase diagram, which every hypoeutectoid steel ultimately intersects as it cools.
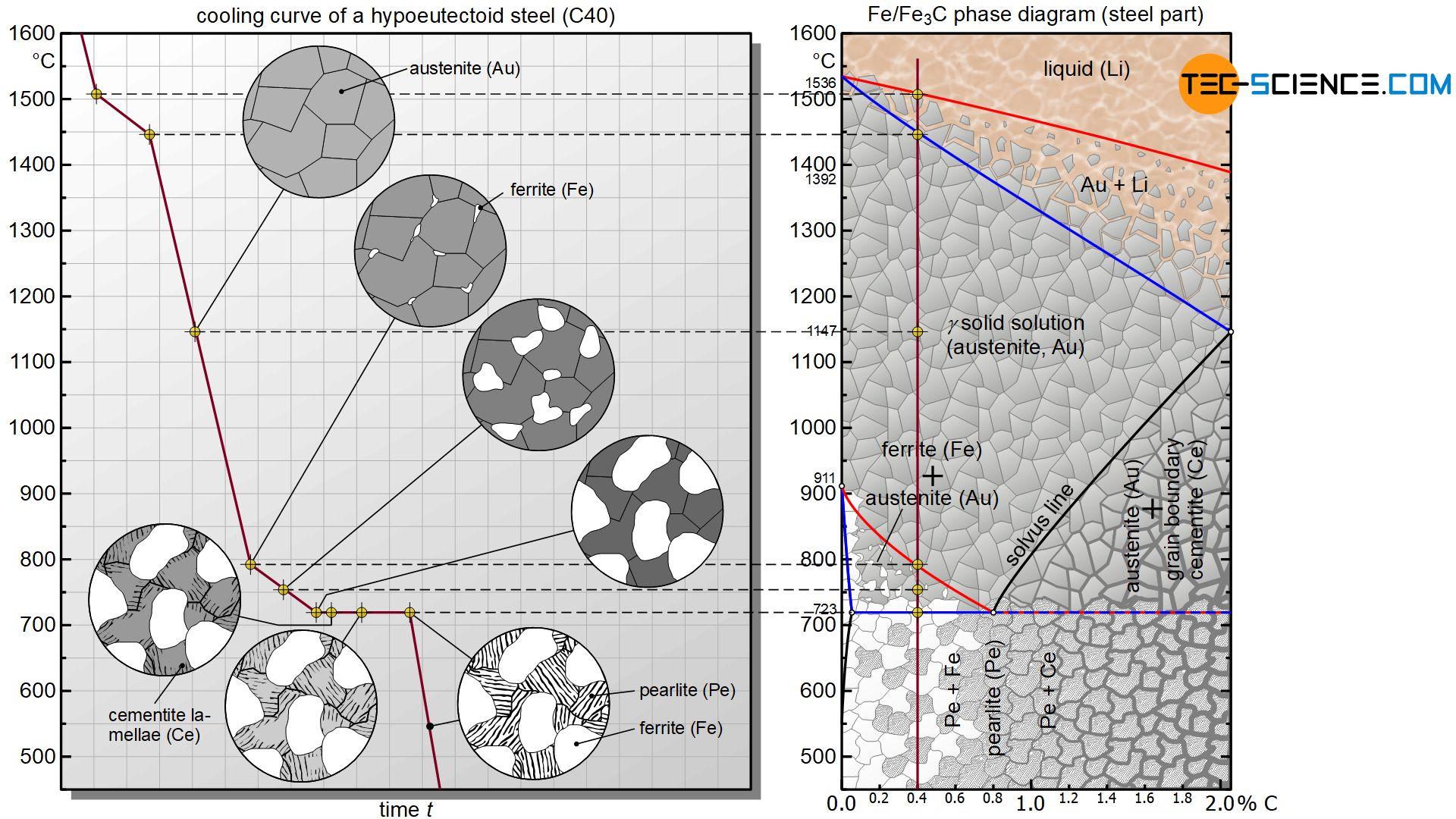
In a hypoeutectoid steel of 0.4 % carbon, the lattice transformation from the face-centered cubic austenite lattice to the body-centered cubic ferrite structure starts at around 800 °C (instead of 911 °C as in the case of pure iron). The face-centered cubic lattice preferably begins to transform into the body-centered cubic microstructure at the energetically favourable grain boundaries. This lattice transformation spreads to the surrounding austenite structure as it cools further.
This transformation no longer takes place at a constant temperature as in the case of pure iron, but in a temperature range. Therefore, the γ-α- transformation again comprises a two phase region in the diagram in which the microstructure consists in parts of the already converted ferrite and the remaining austenite.
In hypoeutectoid steels, parts of the austenite first transform into carbon-insoluble ferrite when cooling!
Since the carbon in the already converted α-iron can no longer be dissolved, it is displaced from the body-centered cubic ferrite. However, the surrounding austenite structure is still able to absorb the segragated carbon due to its undersaturated state.
At the beginning of the lattice transformation, for example, the austenite can absorb up to approx. 1.0 % carbon at 800 °C; however, the considered steel only has a carbon content of 0.4 %. Thus, there is still enough space in the austenite to absorb the displaced carbon atoms. Therefore, the carbon precipitated from the ferrite lattice diffuses into the adjacent retained austenite.
Unlike hypereutectoid steels, carbon is not deposited as cementite at the grain boundaries, but is absorbed by the surrounding austenite during γ-α-transformation!
With further cooling, the ferrite grains grow, so that more and more carbon diffuses into the surrounding austenite grains. This leads to a corresponding enrichment of the carbon content in austenite. The respective concentrations can be determined – as is usual in two-phase regions – after approaching the phase boundary and subsequently drawing a vertical line onto the concentration axis. At 750 °C, for example, the carbon content in austenite has increased to approx. 0.6 %, while the carbon concentration in ferrite is of course 0 % due to neglected solubility.
With further cooling and thus increasing ferrite formation, the carbon in the retained austenite accumulates more and more. At 723 °C, the carbon content finally rose to 0.8 %. The retained austenite has now reached the eutectoid composition and is completely saturated, i.e. it can no longer absorb any more carbon. The retained austenite now behaves like a eutectoid steel and finally begins to convert to the eutectoid pearlite at a constant temperature.
Due to the transformation of ferrite from the austenite, it is enriched with carbon until the eutectoid composition is reached at 723 °C and the retained austenite is converted to pearlite.
Thus, the remaining austenite also undergoes the lattice transformation into the carbon-insoluble ferrite structure. The carbon formerly dissolved in the austenite lattice forms the iron carbide compound cementite, which precipitates in lamellar form from the residual austenite during the lattice transformation.
At room temperature, hypoeutectoid steels have a pearlitic basic structure (ferrite grains with embedded cementite lamellae) with the previously formed ferrite grains!
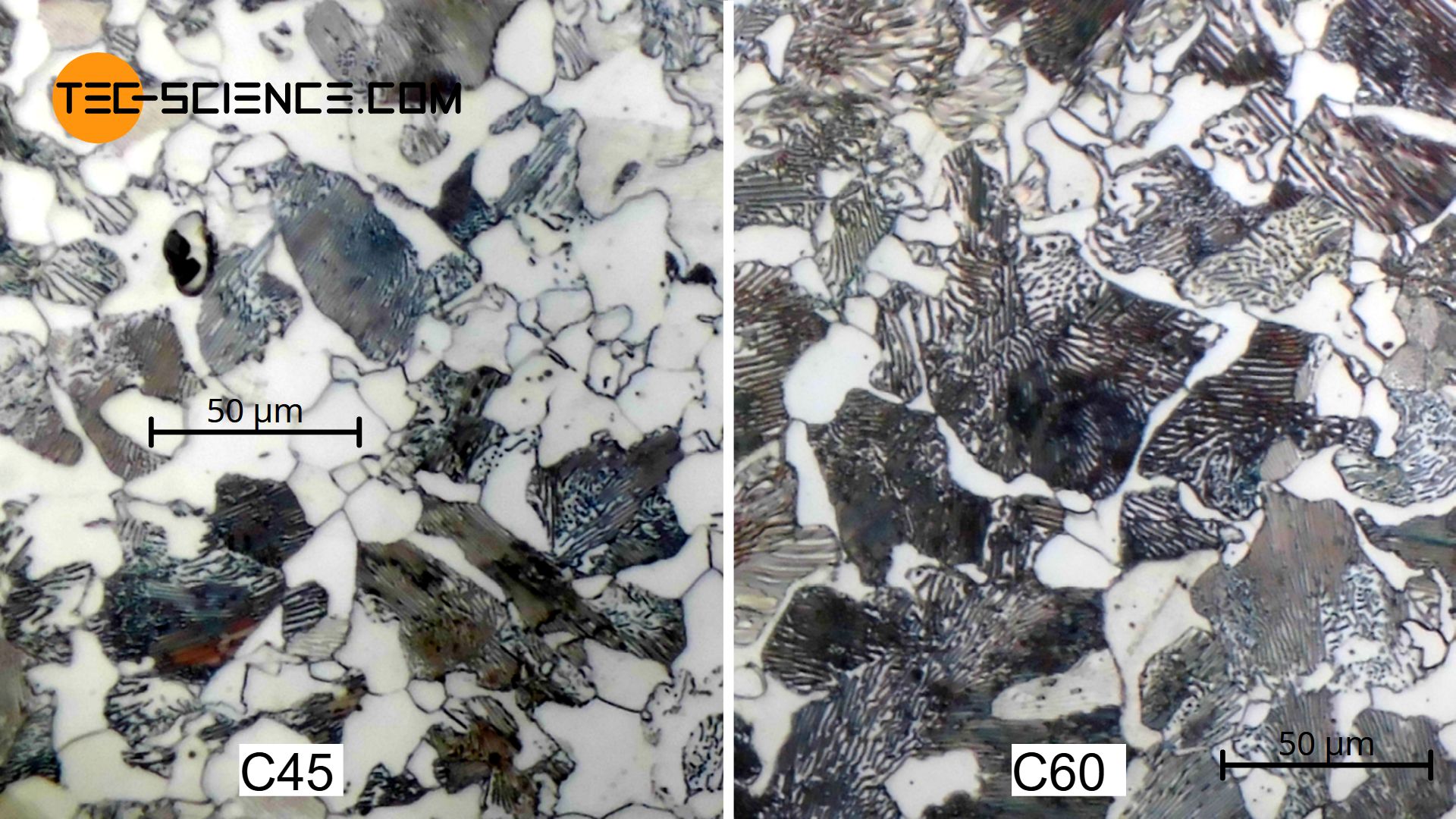
The micrograph below shows a hypoeutectoid steel with 0.45 % carbon (quenched and tempered steel C45). The ferrite grains (white) and pearlite grains (dark stripes) can be seen. In comparison, a microstructure of a hypoeutectoid steel with a higher carbon content of 0.60 % (quenched and tempered steel C60) is shown. Due to the higher carbon concentration, the C60 also has a significantly higher pearlite content in the microstructure.
Note
Note that the γ-α-conversion is always completed at 723 °C regardless of the actual carbon concentration of the steel, as the retained austenite has always reached the eutectoid composition of 0.8 % carbon at this temperature. This applies not only to hypoeutectoid steels but also to hypereutectoid steels!
While for hypoeutectoid steels the carbon concentration in residual austenite accumulates up to 0.8 % carbon due to ferrite precipitation, for hypereutectoid steels the carbon concentration in residual austenite decreases due to cementite precipitation at the grain boundaries until 0.8 % carbon is also reached there. In both cases, this eutectoid composition of retained austenite is reached at a temperature of 723 °C and the retained austenite always decomposes to pearlite at this constant temperature.