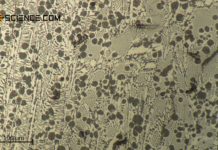
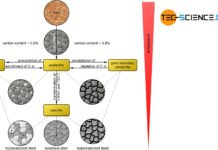
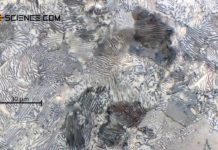
Phase transformations in steels can be compared to those of solid solutions (completely soluble) and crystal mixtures (completely insoluble).
The figure below shows steel part of the iron-carbon phase diagram of the metastable system. A closer look at the transformation lines below the solidus line shows the horizontal “K” typical of alloys, whose components are insoluble in one another.
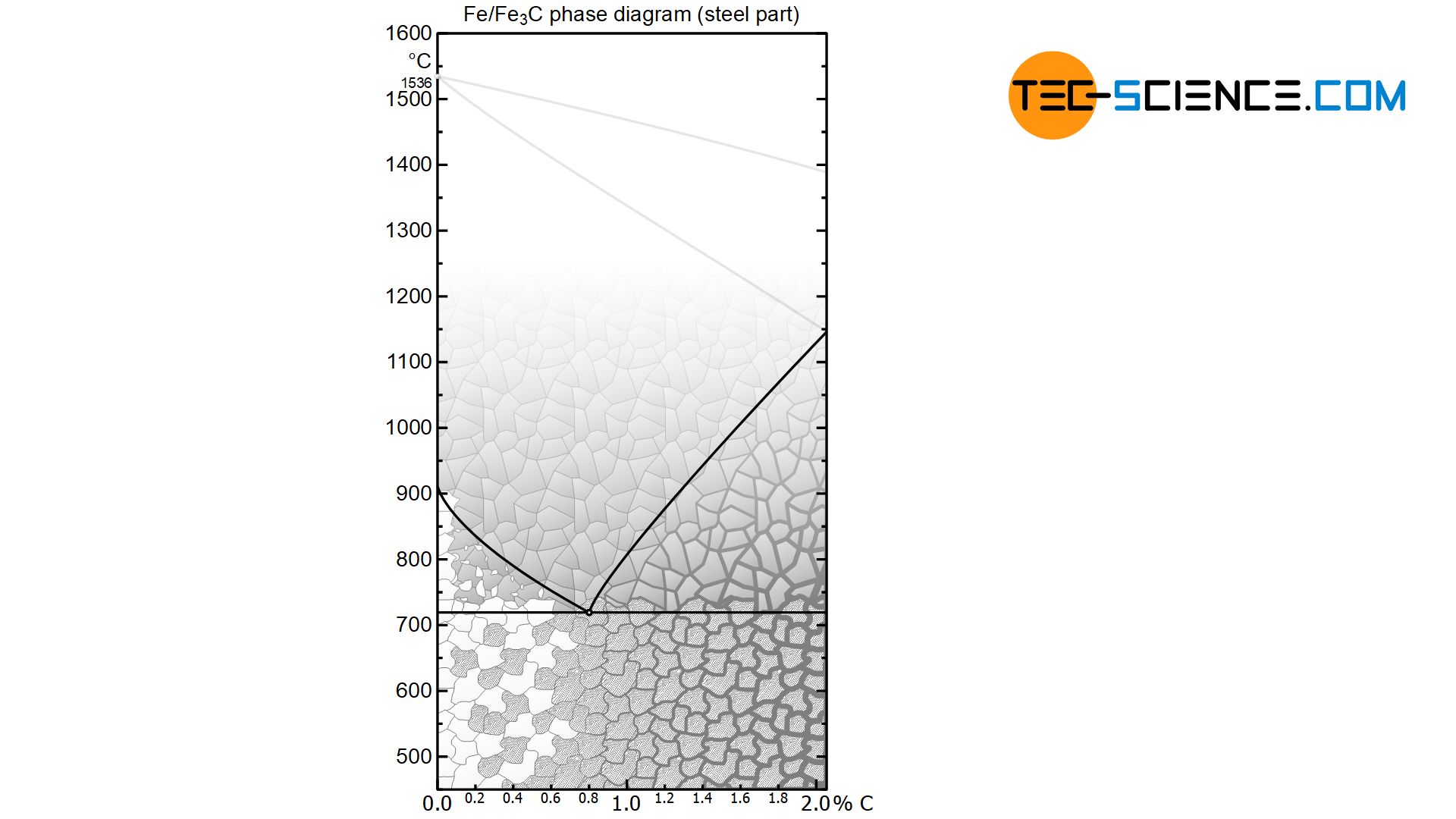
And indeed, the phase transformations of the solidified steel can be considered in analogy to such an alloy, in which the components involved are insoluble in the solid state. After all, the carbon in the \(\alpha\)-iron lattice is also (almost) insoluble at room temperature and is therefore basically a mixed crystal alloy.
The analogy of both phase diagrams and their differences are explained in more detail below.
An essential difference between the two phase diagrams is that the transformation processes in the iron-carbon phase diagram take place not in the liquid state but in the solid state. For this reason, a distinction is made between the terms eutectic (“originating from the melt”) and eutectoid (“originating from the solid state”).
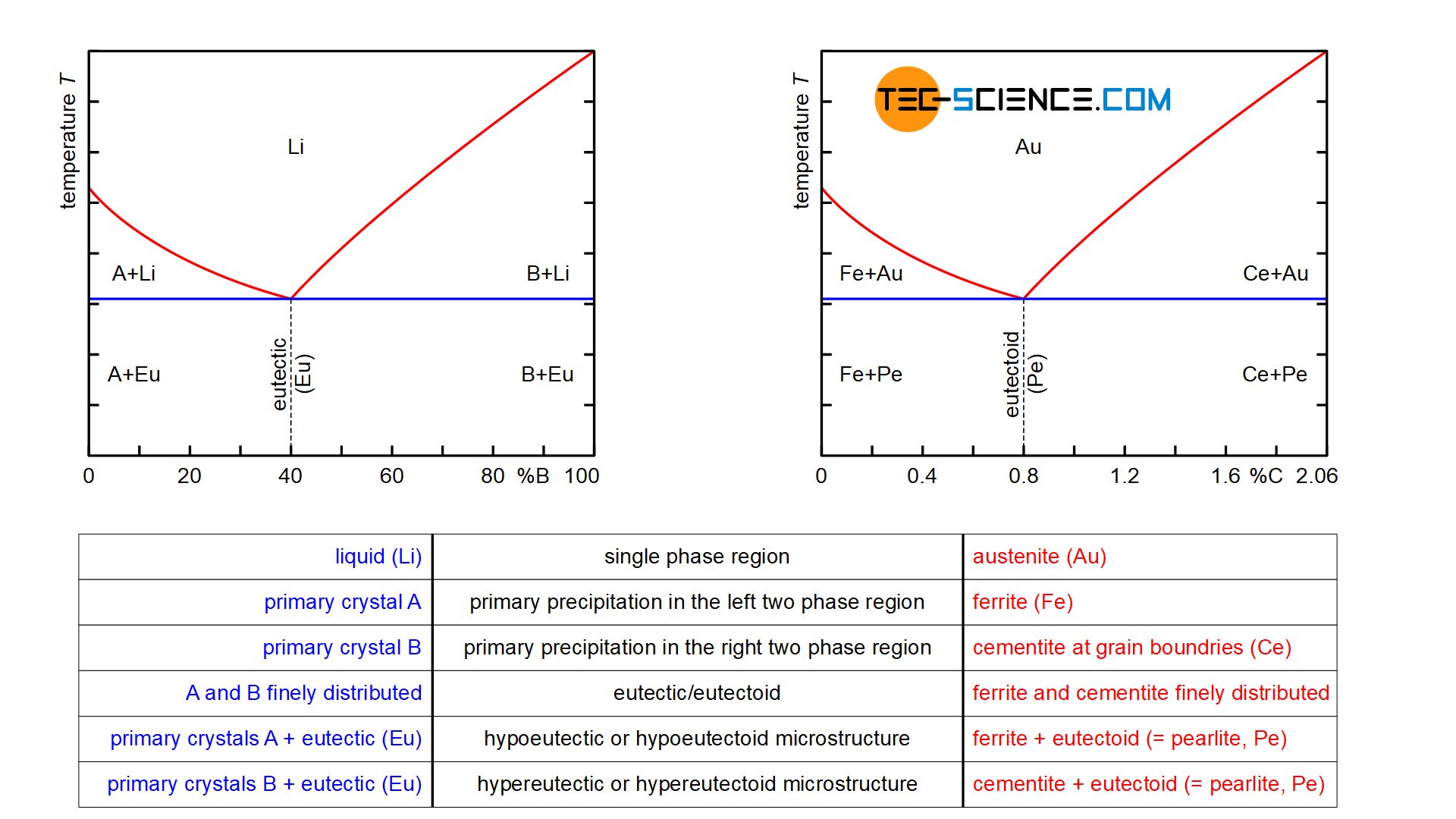
While the A/B crystal mixture alloy is initially present as a homogeneous liquid solution (\(Li\)), the steel is initially also present in the form of the homogeneous solid solution austenite (\(Au\)). These are single-phase regions, as only the melt or austenite is present.
Finally, in the case of hypoeutectic A/B alloys, primary crystals only consiting of atoms (\(A\)) are precipitated when the temperature falls below the corresponding phase line, while ferrite (\(Fe\)) is precipitated in the case of hypoeutectoid steels. These are two-phase regions. Note that the components that are applied to the far left of the concentration axis, i.e. the pure substance \(A\) or pure iron \(Fe\), are precipitated.
Conversely, primary crystals only consisting of B are precipitated in hypereutectic alloys and cementite (\(Ce\)) in hypereutectic steels. Again, these are the components that are applied to the far right of the concentration axis. Note that the phase diagram of the steel was broken off at 2.06 % carbon. Normally the phase diagram on the right ends with 100 % cementite (more on this in the article here).
For hypoeutectic alloys, the residual melt is enriched with B atoms by the precipitation of the primary crystals A until the eutectic composition is finally reached. In the analogous way, the retained austenite is enriched with carbon atoms up to the eutectoid composition by the precipitation of ferrite.
Conversely, in the case of hypereutectic alloys, the precipitation of primary crystals B in the residual melt leads to a reduction in the B concentration down to the eutectic composition. By analogy, in hypereutectoid steels, cementite precipitates at the grain boundaries until the carbon content in the retained austenite has fallen to the eutectoid composition. Both in the case of the A/B crystal mixture alloy and in the case of steels, these precipitation processes take place within the two-phase region at a thermal arrest.
When the eutectic composition in the residual melt is reached, it is finally transformed at a constant temperature into the eutectic, i.e. into a finely distributed mixture of components A and B which are insoluble in one another. In the same way, the retained austenite in steels transforms into the eutectoid pearlite after reaching the eutectoid composition, i.e. into a finely distributed mixture of the components ferrite and cementite, which are insoluble in one another.
In the solidified state, hypoeutectic alloys finally consist of the precipitated primary crystals A and eutectic formed from the residual melt. In hypereutectic alloys, on the other hand, the microstructure contains the precipitated primary crystals B, between which the eutectic is again located. By analogy, the microstructure of hypoeutectoid steels consists of the precipitated ferrite crystals and the eutectoid pearlite, which has formed from the residual austenite. In hypereutectoid steels, however, the microstructure shows the precipitated cementite at the grain boundaries in addition to the eutectoid.