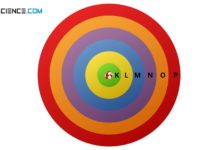
Bohr-Sommerfeld model
The Bohr-Sommerfeld model is an extension of the Bohr model. It explains the distribution of electrons within the shells.
The weaknesses of the Bohr model could be partially eliminated by the physicist...
Bohr’s atomic model
According to the Bohr's atomic model, electrons move on discrete shells around the nucleus (discrete energy levels).
The Rutherford model in many cases provides a very good explanation of physical processes in...
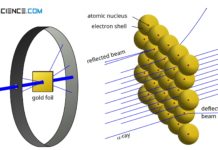
Rutherford’s atomic model
According to Rutherford's atomic model, negatively charged electrons move around a positively charged atomic nucleus.
In 1910, the physicist Ernest Rutherford found that when a thin gold foil was bombarded with α-particles...
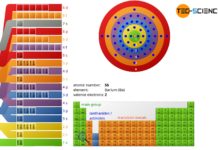
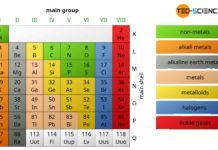
Periodic table of chemical elements
In the periodic table all chemical elements are classified according to their atomic number and their chemical properties.
https://www.youtube.com/watch?v=fxaH5KXQfvc
In the periodic table all chemical elements are classified according to their atomic number...
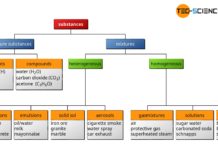
Classification of matter
Substances can be categorized into different groups, such as pure substances or mixtures, depending on their structural composition.
https://youtu.be/v1IVKdgn3So
First, you can distinguish between pure substances and mixtures. Pure substances contain only one...
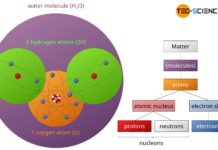
Structure of matter
Matter is made up of microscopic units called atoms. An atom consists of a positively charged atomic nucleus (protons and neutrons) and a negatively charged electron shell (electrons).
https://youtu.be/v1IVKdgn3So
Atomic structure
Matter is made...