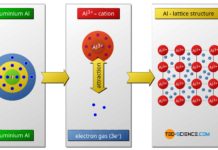
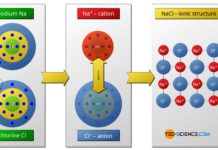
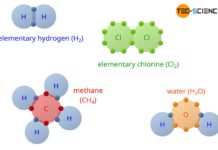
The octet rule refers to the striving of atoms to reach the closest noble gas configuration in the periodic table by forming chemical bonds.
In nature, substances rarely appear as pure elements. Much more, different elements bond for energetic reasons with each other and form chemical compounds. A typical example of this is water (H2O). In this case, two hydrogen atoms (2H) combine with one oxygen atom (O) to form a stable water molecule:
\begin{align}
\label{wasser}
& 2 H ~+~ O ~\rightarrow ~ H_2O \\[5px]
\end{align}
On the other hand, bringing the two hydrogen atoms into contact with an argon atom (Ar) will not result in a stable bonding between these elements. Rather, the argon atom remains for itself and the two hydrogen atoms combine to form elementary hydrogen (H2):
\begin{align}
\label{argon}
& 2 H ~+~ Ar ~\rightarrow ~ H_2 ~+~ Ar \\[5px]
\end{align}
If one considers the chemical elements with regard to their bonding behavior, it is noticeable that the elements of the 8th main group in the periodic table, are particularly stable. They take virtually no chemical reactions with other atoms and therefore do not form molecules. In nature they occur only monatomic (that means as single atoms).
For this reason, the argon atom does not bind chemically with the two hydrogen atoms. The elements of the 8th main group are all gaseous at room temperature, which gives this group the name noble gases. Based on their “sluggish” chemical behavior, these gases are also referred to as inert gases. Since these noble gases practically do not react with other substances, some of them are used as shielding gases against unwanted oxidation during welding.
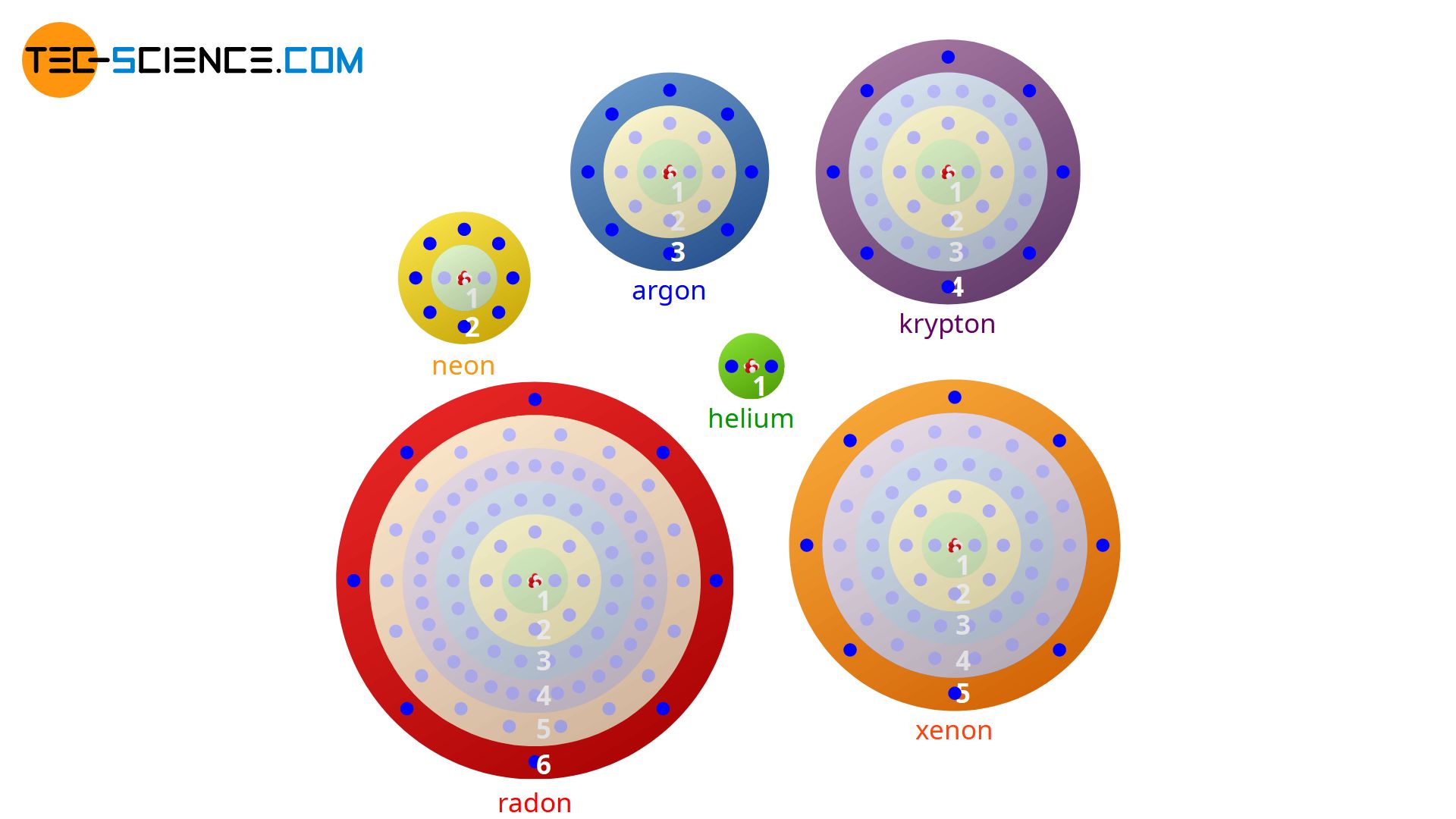
Since the number of outermost electrons decisively influence the chemical behavior of an atom, the number of eight so-called valence electrons (or two in case of helium) means a particularly stable electron occupation. This electron configuration is obviously very favorable in terms of energy. The experimental investigations of the chemical bonding behavior of different atoms also confirm this assumption. This shows that atoms always try to form chemical bonds in such a way that eight or two valence electrons form around the atoms involved.
This state of an atom within a chemical bond with eight or two external electrons is also referred to as an noble gas configuration. Thus, an important rule for the chemical bonding behavior can be derived:
Each atom strives to reach the closest noble gas configuration in the periodic table (octet rule).
Based on the noble gases with their eight valence electrons (exception: helium), the effort to achieve the noble gas configuration is also called octet rule. The noble gas state is achieved by the fact that the atoms form chemical bonds and thereby
- absorb or release electrons (ionic bond, metal bond), or
- use it together with other atoms (covalent bond).
The respective chapters will briefly explain these most important types of bonding. It should always be noted that in reality, bonds can not be sharply limited to a certain type of bond. Rather, chemical compounds have features of different types of bonds.
Important note
in the context of Bohr’s atomic model, it is often incorrectly claimed that the noble gas configuration means a fully occupied outermost shell. This statement is wrong! For in the Bohr model, the maximum number of electrons on the \(n\)th shell results from the following equation:
\begin{equation}
N_{max} = 2 \cdot n^2
\end{equation}
With \(n\) = 3 argon offers space on its outermost third shell for a maximum of \(N\) = 18 electrons. However, argon only has 8 valence electrons on this shell. The outermost shell is therefore far from being fully occupied! At this point it is argued with the wrong atomic model. Rather, the statement of the fully occupied (sub)shell is to be seen in connection with the orbitals that Sommerfeld introduced as an enhancement of the Bohr model (Bohr-Sommerfeld model):
The noble gas configuration means a fully occupied orbital (subshell) in the Bohr-Sommerfeld model!
More specifically, the noble gas configuration means a fully occupied p orbital. The exception is helium with a fully occupied s orbital.