Matter is made up of microscopic units called atoms. An atom consists of a positively charged atomic nucleus (protons and neutrons) and a negatively charged electron shell (electrons).
Atomic structure
Matter is made up of microscopic units called atoms. Chemical elements are composed of atoms of a certain type. The classification of the elements takes place according to the periodic table. If several atoms (chemical elements) react with each other and form a stable unit by chemical bonds, these are called molecules.
Molecules are stable compounds of atoms by using chemical bonds.
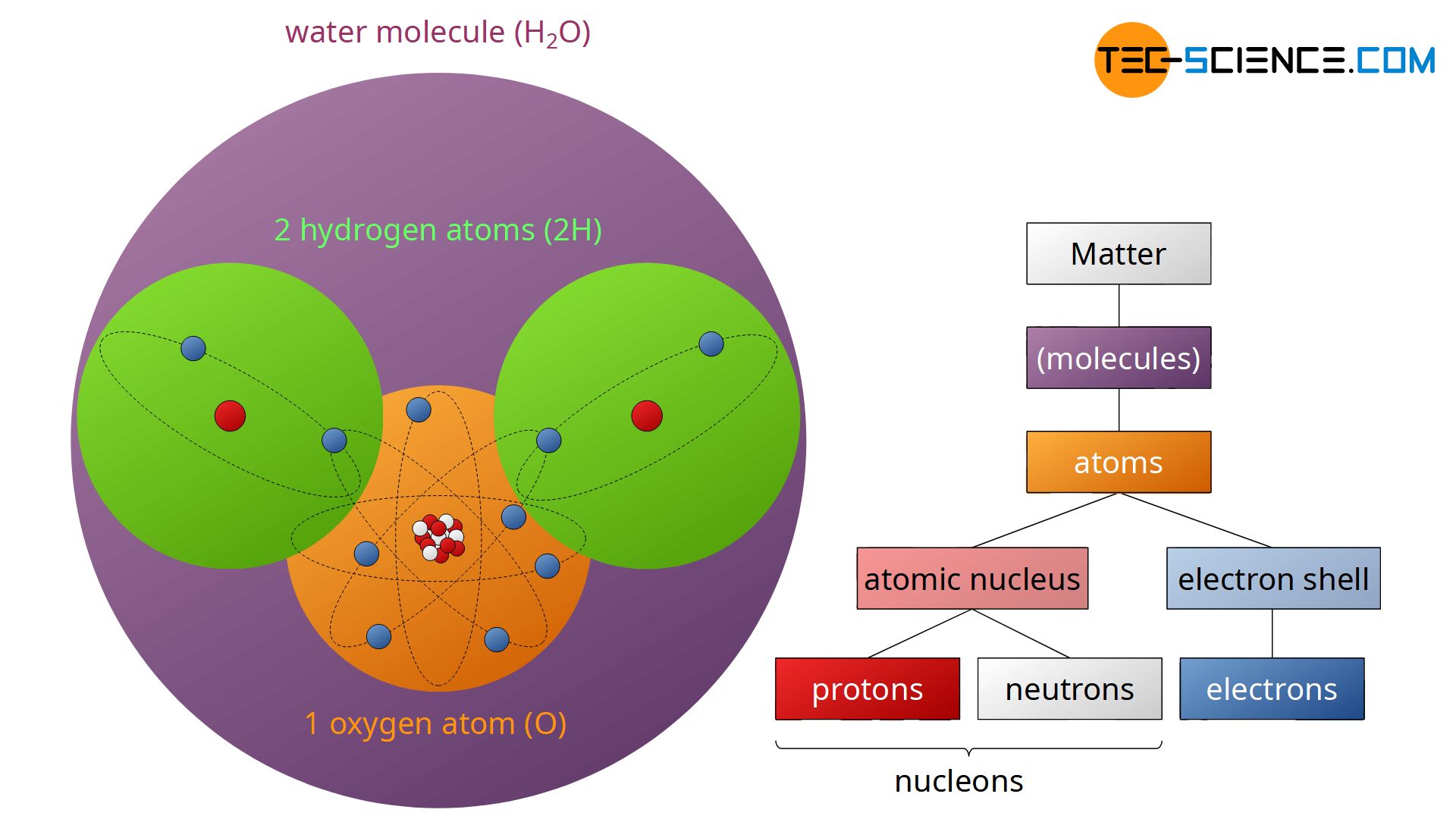
For example, water consists of the elements hydrogen and oxygen. In each case, two hydrogen atoms (H) and one oxygen atom (O) join together in order to form a stable H2O molecule. Atomic units such as molecules, atoms, protons, neutrons, electrons, etc. are simply referred to as particles. In this connection one often speaks of the so-called particle model of matter.
The particle model of matter means that matter is made up of particles without making a difference between atom, molecules, etc.
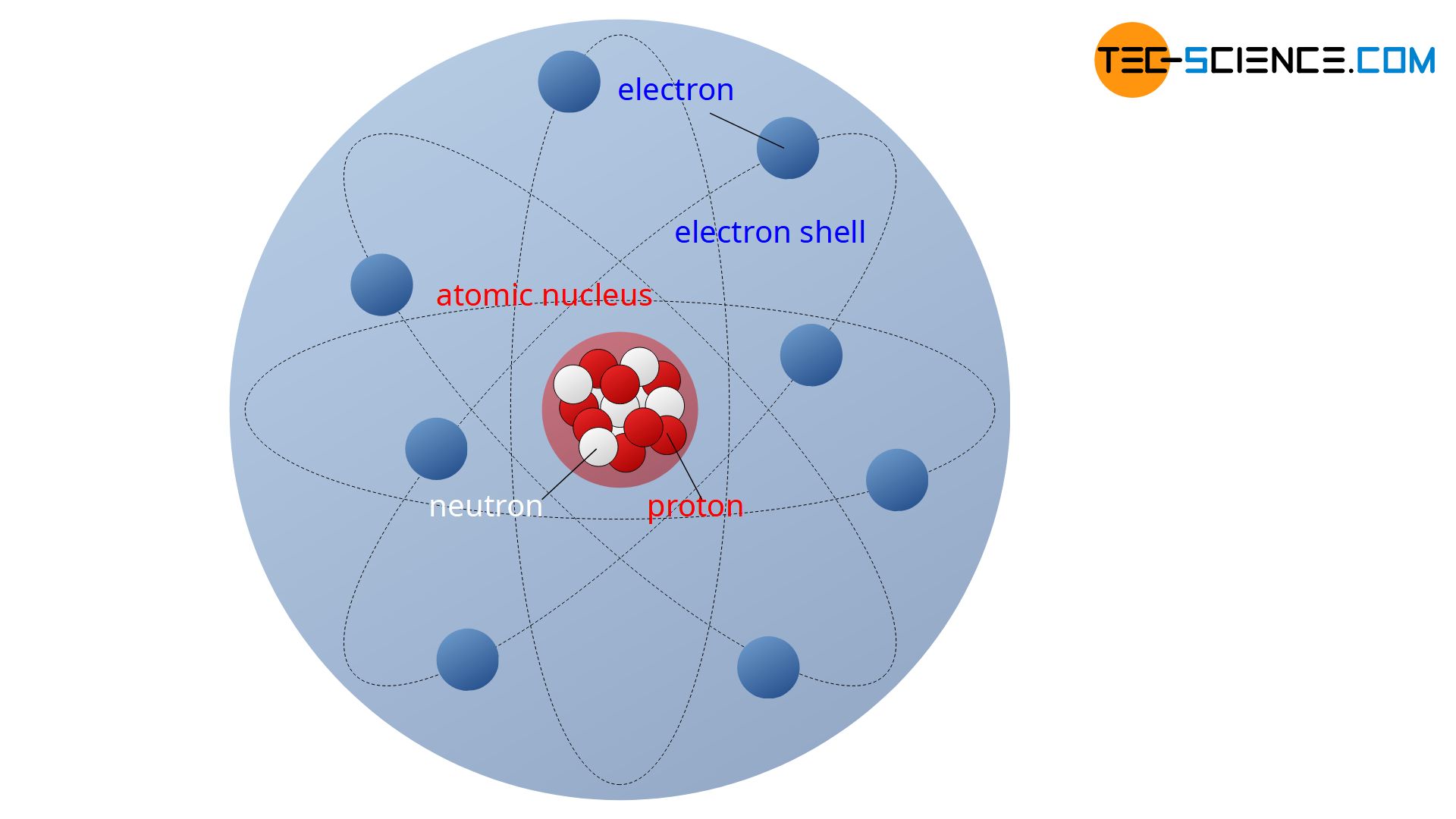
According to Rutherford’s atomic model, an atom consist of an positively charged atomic nucleus (lat. nucleus = “core”) and an negatively charged electron shell. The nucleus contains positively charged protons. These protons are the reason for the positive charge of the atomic nucleus.
The repulsive force between the protons due to their identical charges is compensated by the strong attraction of the neutrons, who are also present in the atomic nucleus. The neutrons themselves are electrically neutral, but they still exert a strong attraction to the protons. In this way the protons are held together stably in the nucleus.
The particles in the nucleus (neutrons and protons) are also referred to as nucleons.
The attraction between the protons an the neutrons can not be caused by a electrostatic field because neutrons do not carry electric charges and theirfore can not be affected by such a field. It is rather another type of force. This force is called strong nuclear force or strong interaction. In addition to the electromagnetic force, the gravitational force (gravity) and the weak interaction (weak nuclear force), the strong interaction is one of the four fundamental forces of physics.
The interaction of the strong nuclear force is very limited in range, but at small distances as in atomic nuclei , that force can be extremely strong. The strong interaction between the protons and neutrons is the reason why this nuclear force outweighs the repulsive electrostatic forces of the protons and thus holds the nucleus together. The strong interaction is the “clue” for the nucleus so to speak.
The strong nuclear force (strong interaction) between the nucleons holds the atomic nucleus together.
The electron shell is located around the positively charged nucleus of an atom. It is formed by the negatively charged electrons. In a simplistic notion, the electrons in this imaginary shell orbit the positive nucleus. The electrostatic forces of attraction between the positive nucleus and the negative electrons ensure that the orbiting electrons are held stably on their path around the atomic nucleus, so that the atom does not fall apart.
The electron shell is an imaginary shell where the electrons orbit the nucleus.
Atomic number
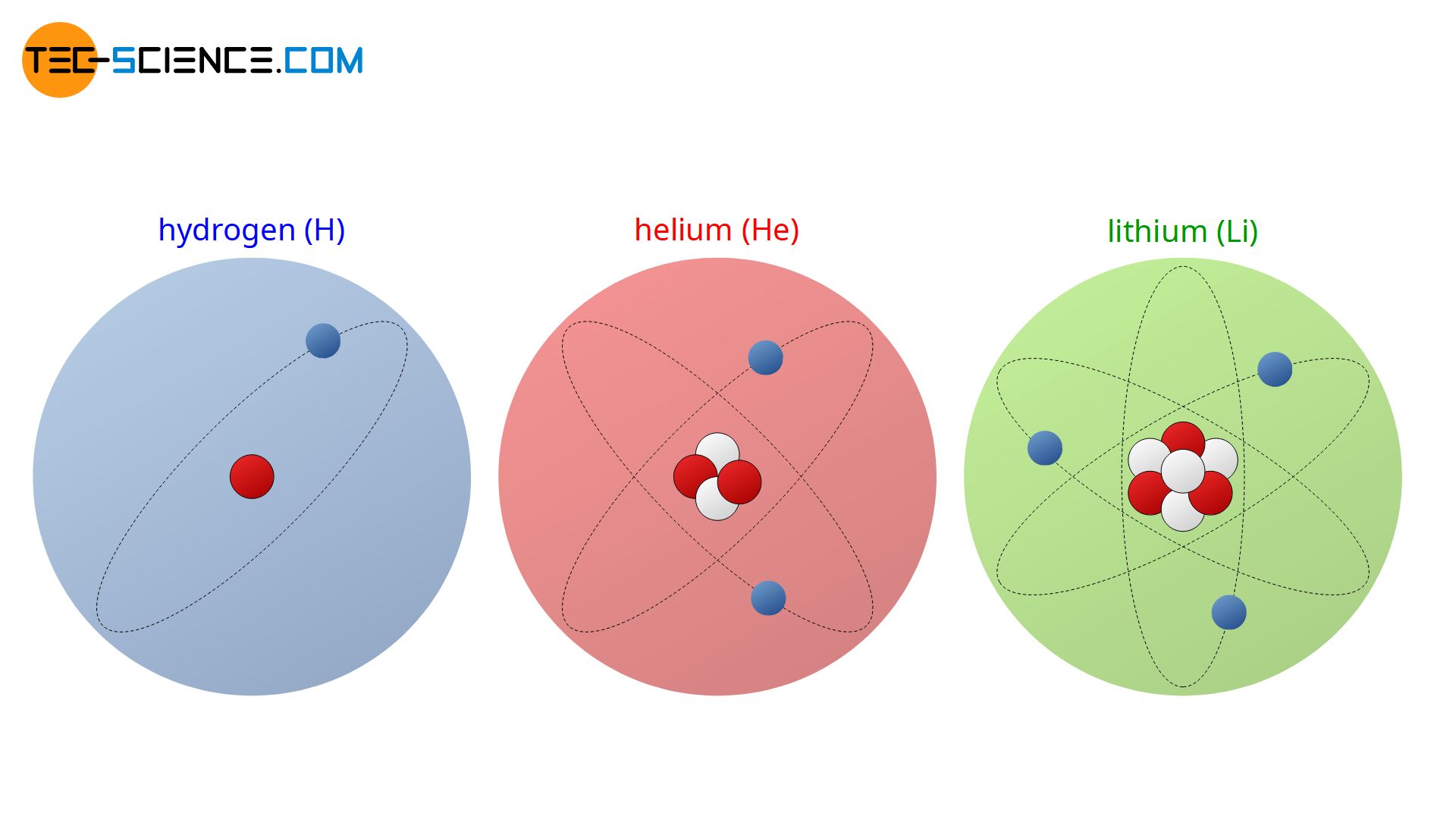
Characteristic for a particular type of atom or for a chemical element is the number of protons in the nucleus! The number of protons essentially determines the chemical behavior of the element and is responsible for the order in the periodic table. Therefore, the number of protons is often referred to as atomic number. For example, a hydrogen atom always has one proton in its nucleus. If it contained two or three protons in the nucleus, it would no longer be a hydrogen atom but a helium atom (2 protons) or a lithium atom (3 protons).
The number of protons in an atom’s nucleus (atomic number) determines the element.
Isotopes
In contrast to the number of protons, the number of neutrons is not characteristic for a chemical element. For example, a lithium atom usually has four neutrons in its nucleus. However, this only applies to 92.5% of all lithium atoms. The remaining 7.5% contain only three neutrons in the nucleus. Such modifications of atoms with different numbers of neutrons but of course still having the same number of protons (otherwise it would be another element) are referred to as isotopes. The lithium atom thus has two (stable) isotopes.
Isotopes do have the same number of protons but different number of neutrons.
The hydrogen atom even has three isotopes. The most abundant hydrogen isotope (99.98%) has only one proton in its nucleus and no neutron. Becaus of only having one proton that hydrogen isotope is also called protium (symbol: H).
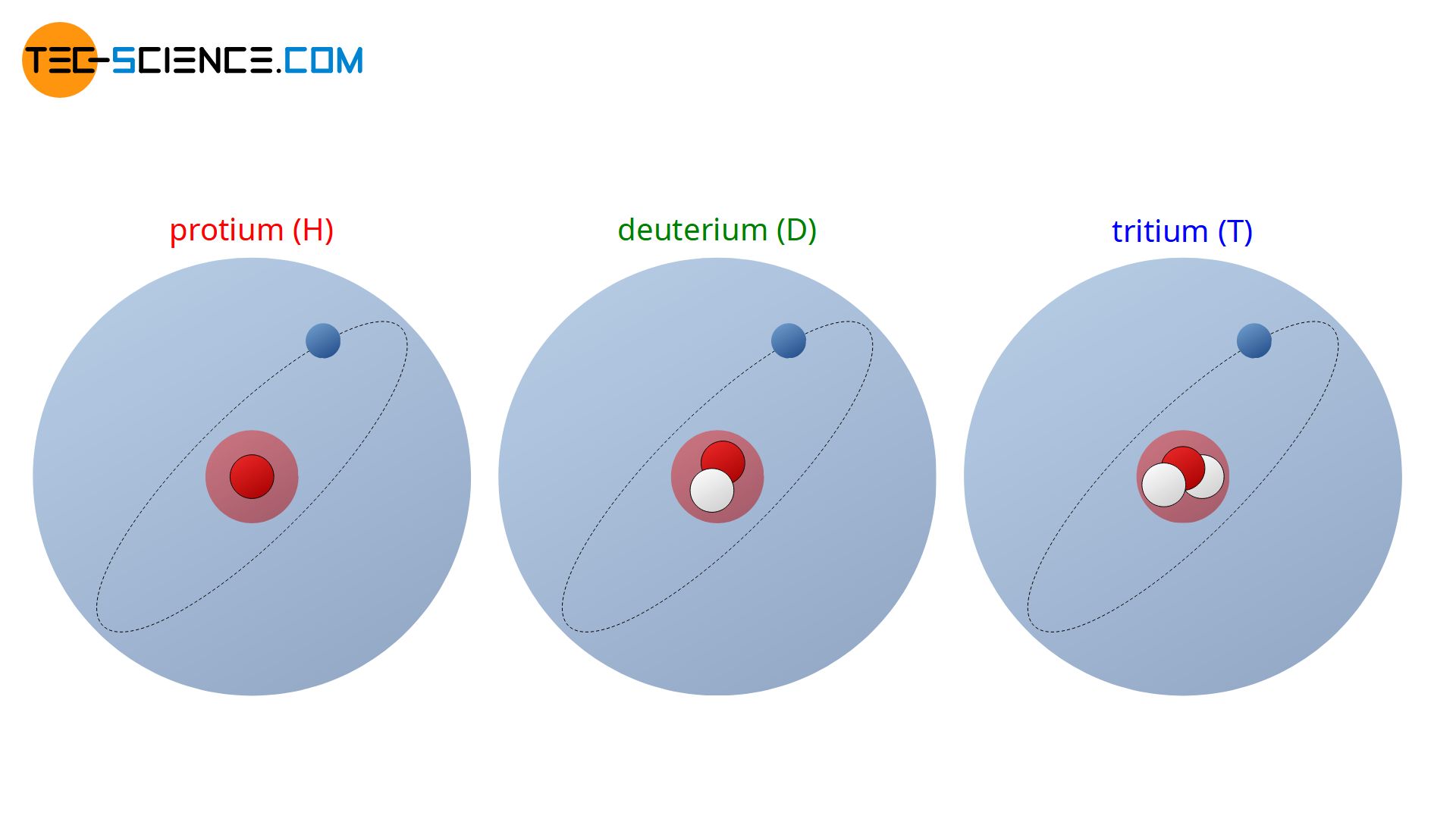
If the hydrogen atom has a neutron in addition to the proton, this isotope is called deuterium (symbol: D). Deuterium is represented by only 0.015% of all naturally occurring hydrogen atoms.
Another hydrogen isotope even has two neutrons in the nucleus and is called tritium (symbol: T). This isotope accounts for only a tiny fraction of total hydrogen in nature. However, unlike protium and deuterium, tritium is not stable and decomposes with a half-life of approximately 12 years. Due to the decomposition tritium is radioactive.
Ions
In the electrically neutral state, there are just as many positively charged protons in the nucleus as there are electrons in shell. The electric charge of an electron and a proton is identical in magnitude, but with the opposite sign. In the macroscopic point of view, the electrostatic effects cancel each other out. In this state, the particle is electrically neutral. However, if this neutral state is disturbed by absorbing or removing electrons, the atom is called an ion. The process of absorbing or losing electron is called ionization.
With excess of electrons, the atom is negatively charged. A negatively charged ion is referred to as an anion. An electrically positively charged ion is called a cation. Since the number of protons determines the element, an ion can only be obtained by donation of electrons, but not through the release of a proton (changing the number of protons would create a completely different element).
An ion is an electrically charged atom (or a group of atoms). A negatively charged atom is called anion and a positively charged atom is called cation.
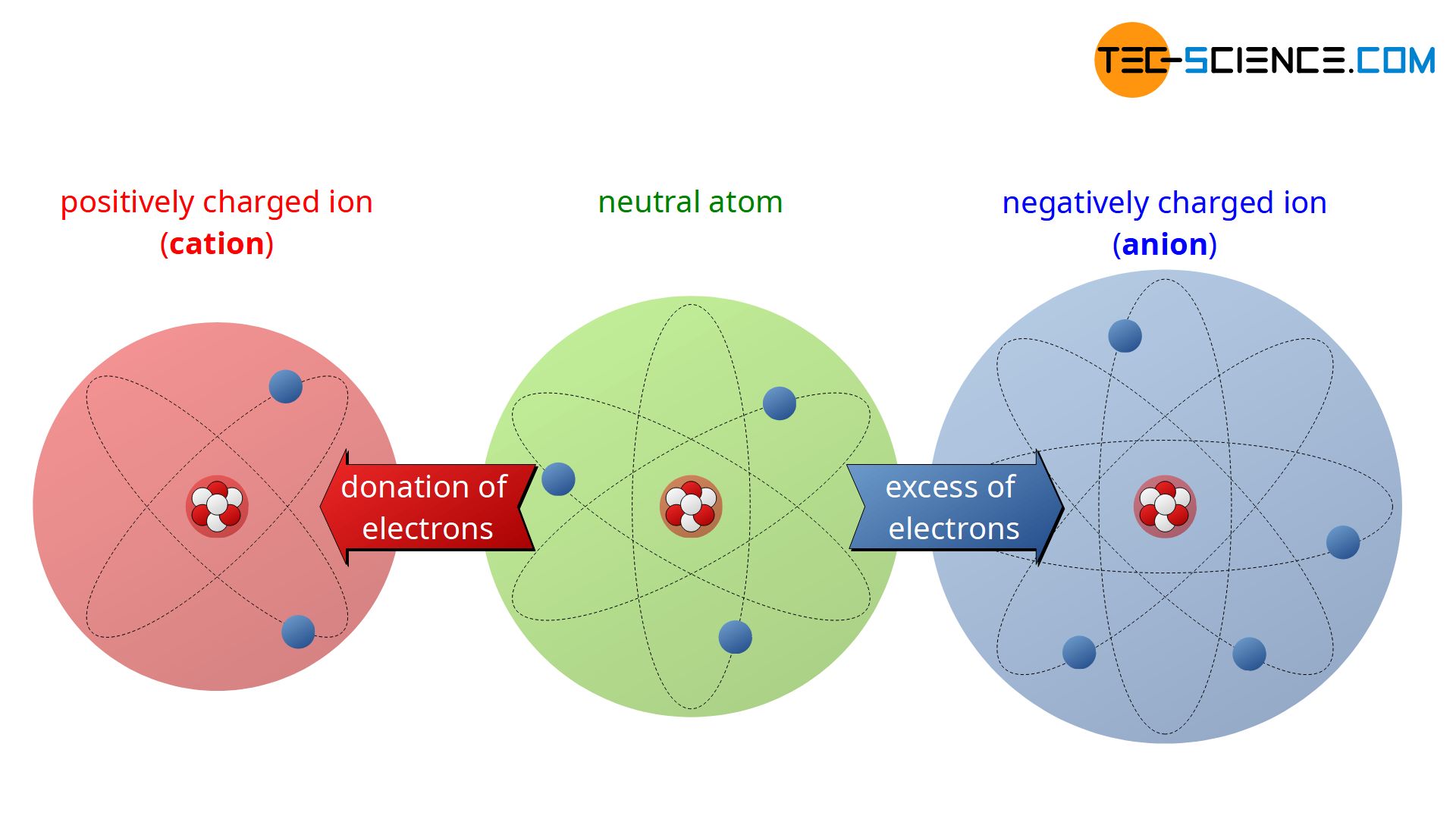
Note, that anions are larger in size than their respective atom due to the excess of electron and cations are smaller in size because of the loss of electrons.