In the periodic table all chemical elements are classified according to their atomic number and their chemical properties.
In the periodic table all chemical elements are classified according to their atomic number and their chemical properties in main group elements (columns IA to VIII A) and transition group elements (IB to VIII B). The number of protons increases continuously from left to right. In addition to this horizontal classification, the periodic table is divided vertically into periods. These periods are not chosen randomly but correspond in the shell model to the electron shell introduced by Bohr (K, L, M, N, O, P and Q).
Elements in a certain group are all showing similar chemical behavior due to their identical amount of electrons in their outermost shell (this accounts only for elements in the main group).
The periodic table arranges elements into groups with similar chemical properties and periods with identical number of shells!
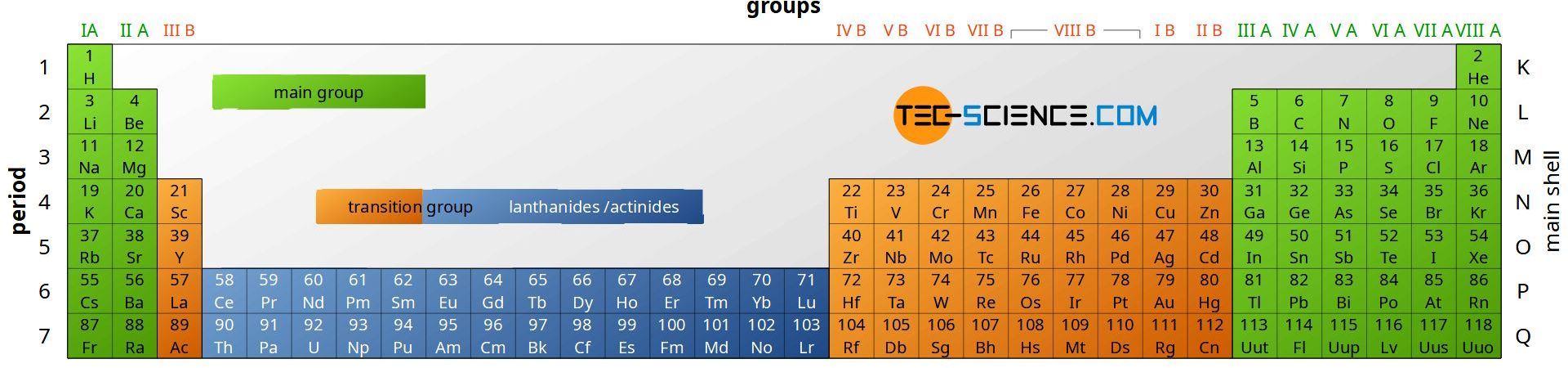
As the number of period increases a new electron shell is added. Therefore the atoms within a certain group grow larger from top to bottom of the periodic table. On the other hand the atomic radius decreases from left to right oft the periodic table. This is due to the increasing number of protons that comes along with the atomic number. The number of electrons in the shell will increase as well . The more protons an atomic nulceus contains the higher its charge and the higher the charge of the electron shell. However, a higher charge results in an stronger force of attraction between the nucleus and the shell. Since the number of shells will not increase within a period the stronger force of attraction will bind the shell much stronger to the nucleus.
The size of an atom will increase within a group from top to bottom but will decrease within a period from left to right!
Within each period the element on the rightmost side of the periodic table will have the highest force of attraction between its nucleus and its shell. This configuration makes the element extremely stable. Since the elements on the rightmoste side are gaseous they are referred to as noble gases (or inert gases).
The classification of the periodic table into main groups and transition groups is due to their different distribution of electrons in atomic orbitals (electron configuration). For the same reason a further division can be made into lanthanides and actinides (the term actionide derives from the fact that all these elements are radioactive).
In the main group the s and p orbitals of the respective atoms are occupied by electrons (“s-block” or “p-block”), while in the transition group an electron is added in the d-orbital of the respective atom (“d-block”). In case of lanthanides and actinides the occupation of the f-orbital (“f-block”) takes place.
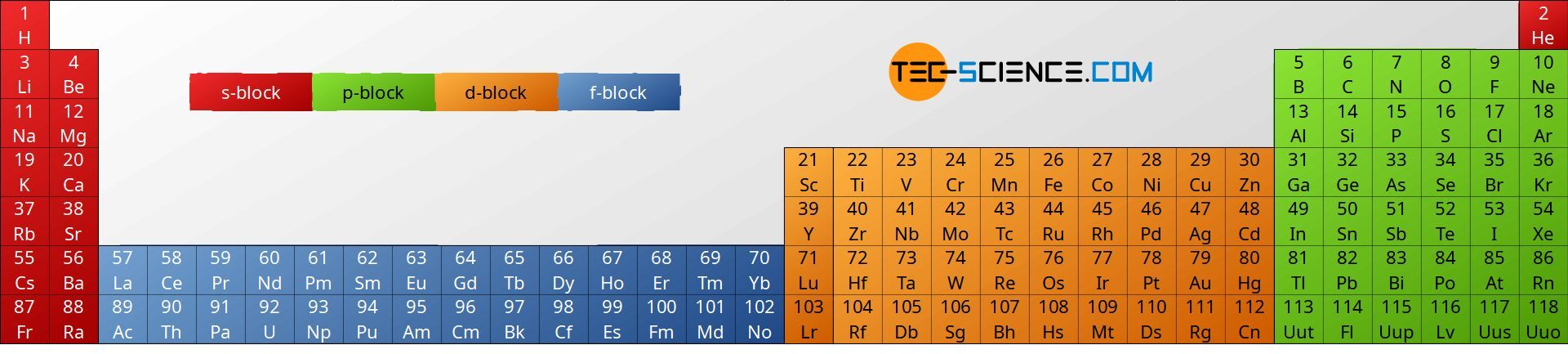
The main group elements can be further subdivided according to their physical and chemical behavior. This is usually done as follows:
- non-metals
- alkali metals
- alkaline earth metals
- metals
- metalloids (sometimes misleading called semimetals)
- halogens and
- noble gases.
Note that alkali metals and alkaline earth metals are “metals” in the true sense. Between the group of the alkaline earth metals and the metals is the transition group which is not shown in this figure. The reason for the word “transition” now becomes clear and since all the elements within the transition group are metals they are also referred to as transition metals. Thus, about 80 % of the existing elements are metals!
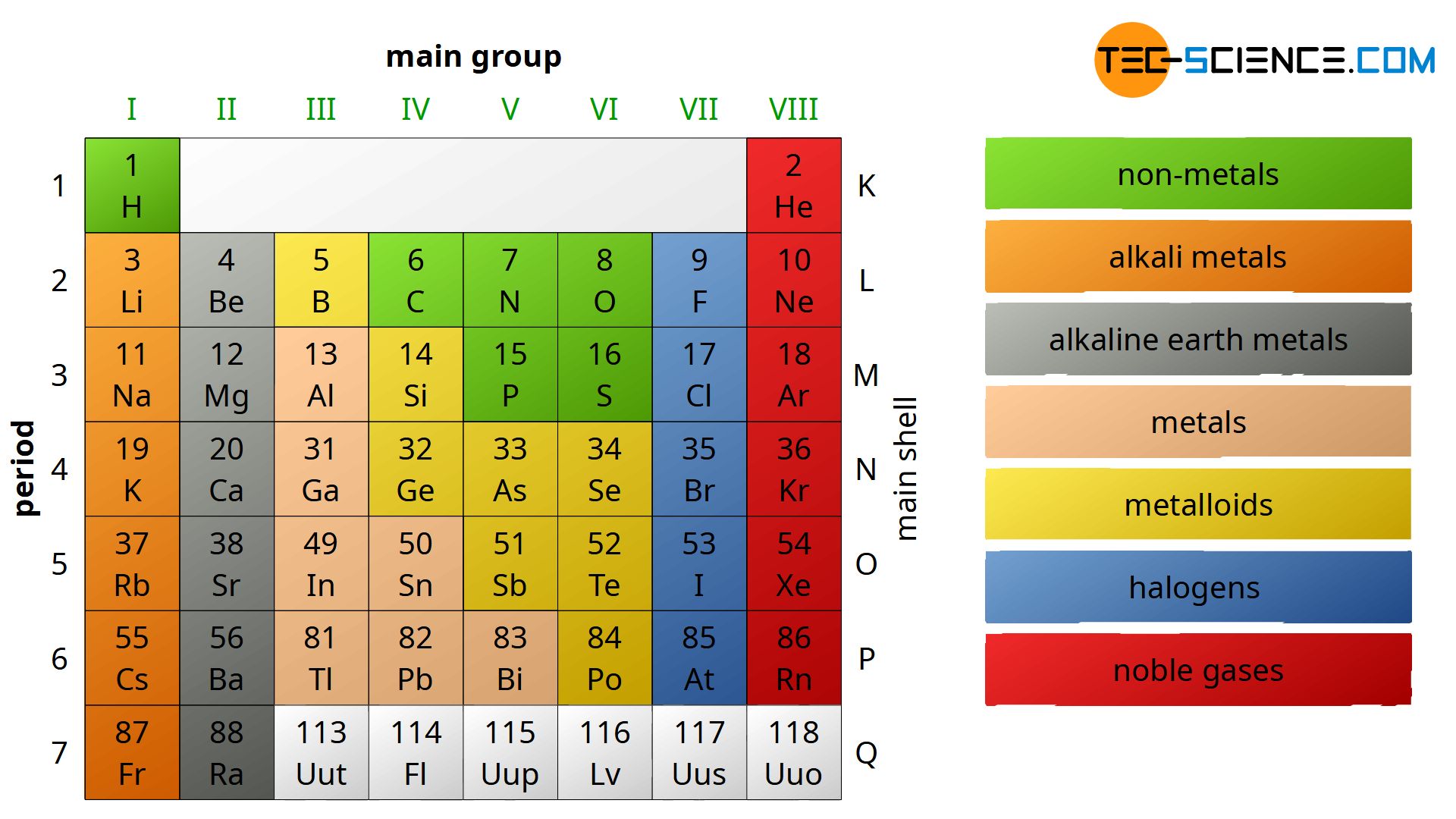
A few elements have proporties of metals as well as of nonmetals. Those are referred to as metalloids. However, there is no cear definition of a metalloid! Usually the metalloids include:
- boron (B)
- silicon (Si)
- germanium (Ge)
- arsenic (As)
- antimony (Sb)
- bismuth (Bi)
- selenium (Se)
- tellurium (Te)
- polonium (Po)
The number of outer electrons of an atom (also referred to as valence electrons) significantly determines the chemical properties of the respective element. For main group elements, the number of valence electrons corresponds directly to the main group number. For example, potassium (Ka) belongs to the main group number 1 and therefore has one electron in its outer shell; so does sodium (Na) and cesium (Cs). Accordingly to the fifth main group, elements like nitrogen (N), phosphorus (P) and arsenic (As) do have five valence electrons.
The number of the main group corresponds to the number of valence electrons of the elements assigned therein! The chemical behavier is mostly influenced by the number of valence electrons!
The chemical similarity due to the common number of valence electrons is particularly evident in the case of the alkali metals (1st main group, with the exception of hydrogen), the alkaline earth metals (2nd main group), the halogens (7th main group) and the noble gases (8th main group) ,
However, this relatively simple determination of the valence electrons based on the group number only works for the main group elements. In the transition group, however, this principle fails. Thus, the entire transition metals have only one or two outer electrons. Consequently, all transition elements have similar chemical properties.