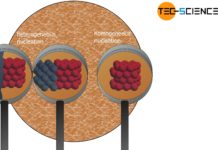
In polygonal crystal growth, the heat of solidification is transferred through the crystal and in dendritic crystal growth through the melt.
Introduction
In the article homogeneous nucleation or heterogeneous nucleation, the formation of nuclei was explained in more detail. If such a stable nucleus emerges from the undercooled melt, then further atoms will accumulate from the melt (by releasing crystallisation heat). The nucleus now begins to grow and initiates the stage of nucleus growth or crystal growth.
The particles are aggregated by diffusion processes through the melt, where they hit the surface of the solidified crystal. The atoms diffuse there at energetically favourable locations. The direction of heat dissipation at the solidification front has a decisive influence on the shape of the growing crystal.
Depending on whether the heat of crystallisation is dissipated via the solidified crystal or the adjacent melt, a distinction is made between polygonal crystal growth and dendritic crystal growth. These two types of crystal growth are described in more detail below.
Polygonal crystal growth
If, for example, a nucleus forms on the surface of a mold wall, the heat of crystallisation released at the growth front is often dissipated via the solidified crystal and then via the mold wall.
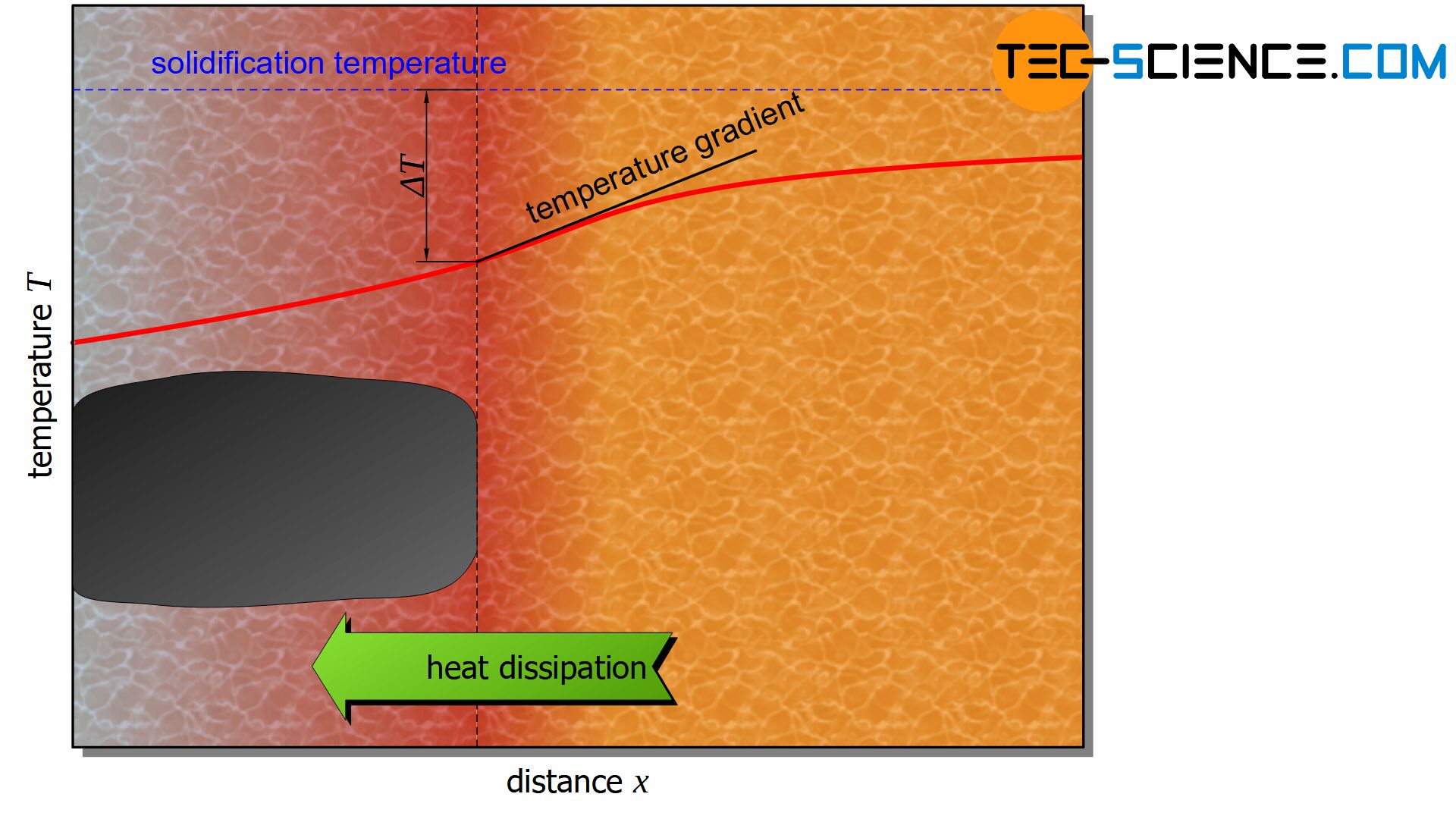
This is due to the fact that the melt generally has a higher temperature than the mold wall and heat dissipation finally always takes place in the direction of decreasing temperature (positive temperature gradient in the direction of the melt).
This is also the case when the melt is relatively slightly supercooled. The crystal then grows in an area with a higher temperature than itself. A possible (premature) branching of the crystal would then melt again. Therefore, the solidification front remains relatively flat. This is also referred to as a stable growth front.
Depending on how strong the heat dissipation via the crystal is, rather roundish crystals (globulites) or elongated crystals grow (columnar crystals).
In polygonal (stable) crystal growth, the heat of crystallization is dissipated via the crystal itself.
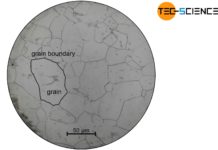
Excursus: Microstructure of a casting
Polygonal crystal growth also explains the typical three-zone microstructure of a solidified casting block (primary microstructure). Such a casted block is also called ingot. In the vicinity of the mold wall, a very fine-grained structure with roundish grains (globulites) forms due to the strong isotropic undercooling caused by the cool mold wall (zone I).
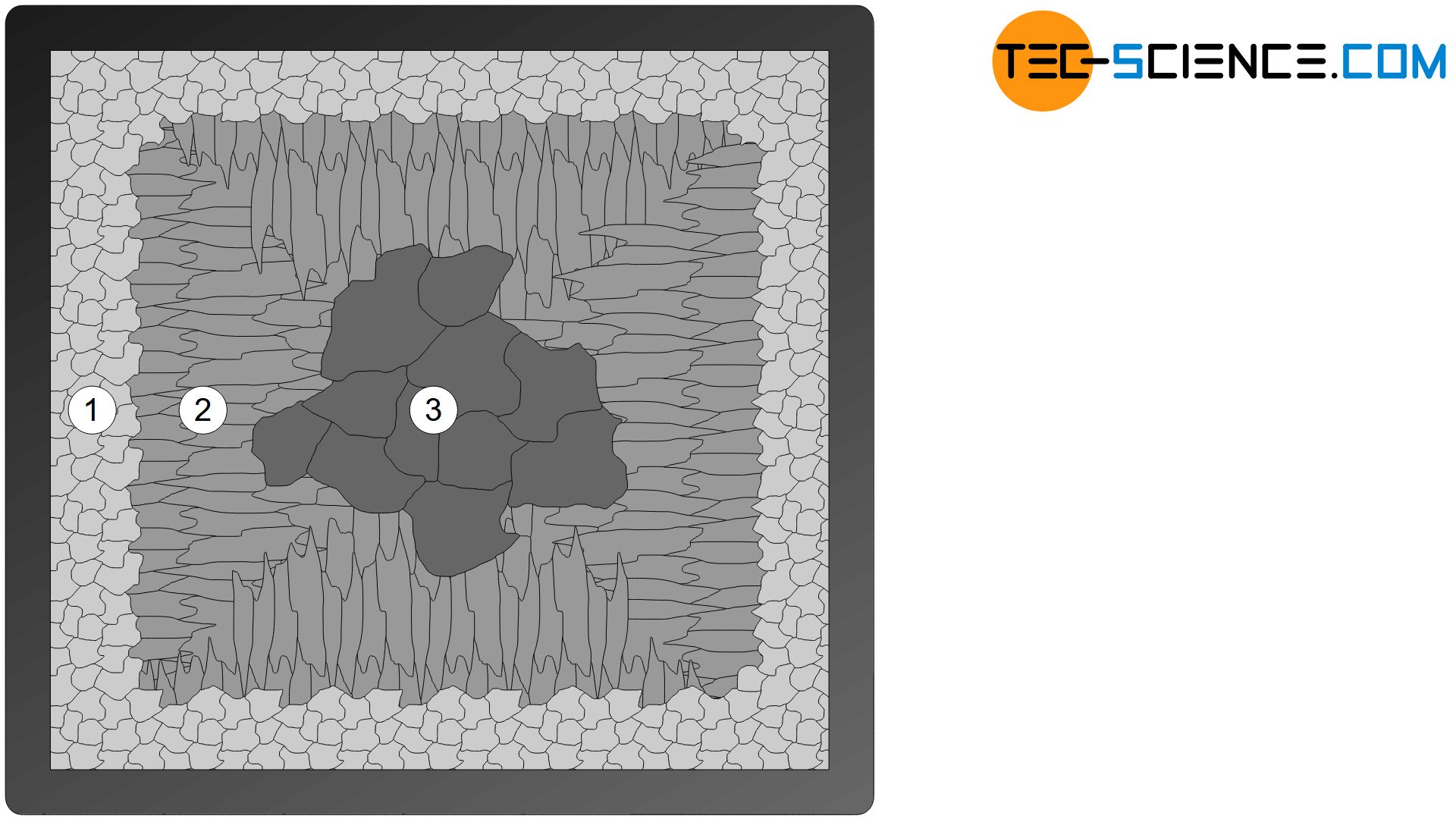
Subsequently, a zone with elongated grains (columnar crystals) is obtained due to the strongly directed, anisotropic heat dissipation towards the mold wall (transcrystalline zone II). The relatively low (isotropic) cooling or undercooling inside the casting block produces a very coarse-grained microstructure (zone III).
By subsequent heat treatment, this heterogeneous structure can be transformed into a homogeneous structure with the desired properties (secondary microstructure).
Dendritic crystal growth
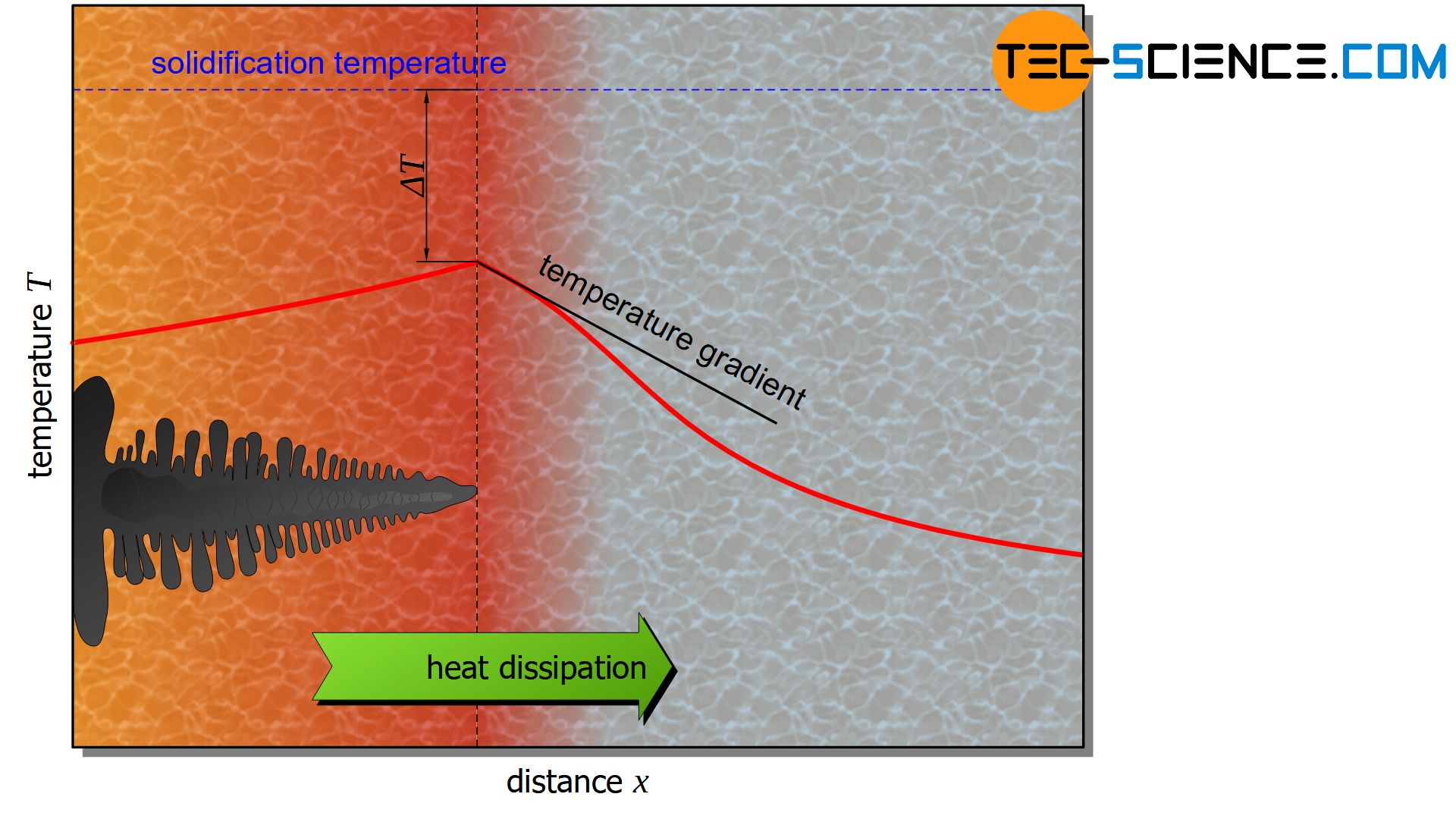
In the case of dendritic crystal growth, heat is not dissipated across the crystal as is the case with polygonal crystal growth, but over the melt.
This will be the case when the melt is severely supercooled and the crystal forms freely in the melt. This results in a negative temperature gradient in the direction of the melt. The crystal now grows in an area with a lower temperature than itself.
Any (advance) branching that may form will continue to grow very quickly, as the cooler melt crystallises rapidly at the branching. The crystal forms branches that are very reminiscent of a fir-tree like structure – these are called dendrites.
In dendritic (unstable) crystal growth, the heat of crystallization is dissipated via the surrounding melt.
The micrograph below shows copper with an increased oxygen content. During solidification, the bluish shimmering copper (I) oxide Cu2O (also called copper oxide) formed, which shows dendritic growth. Due to the microstructure cut, only the dendrite branches cut in the plane can be seen. The dendrites are embedded in the eutectic matrix with finely distributed copper and copper oxide.
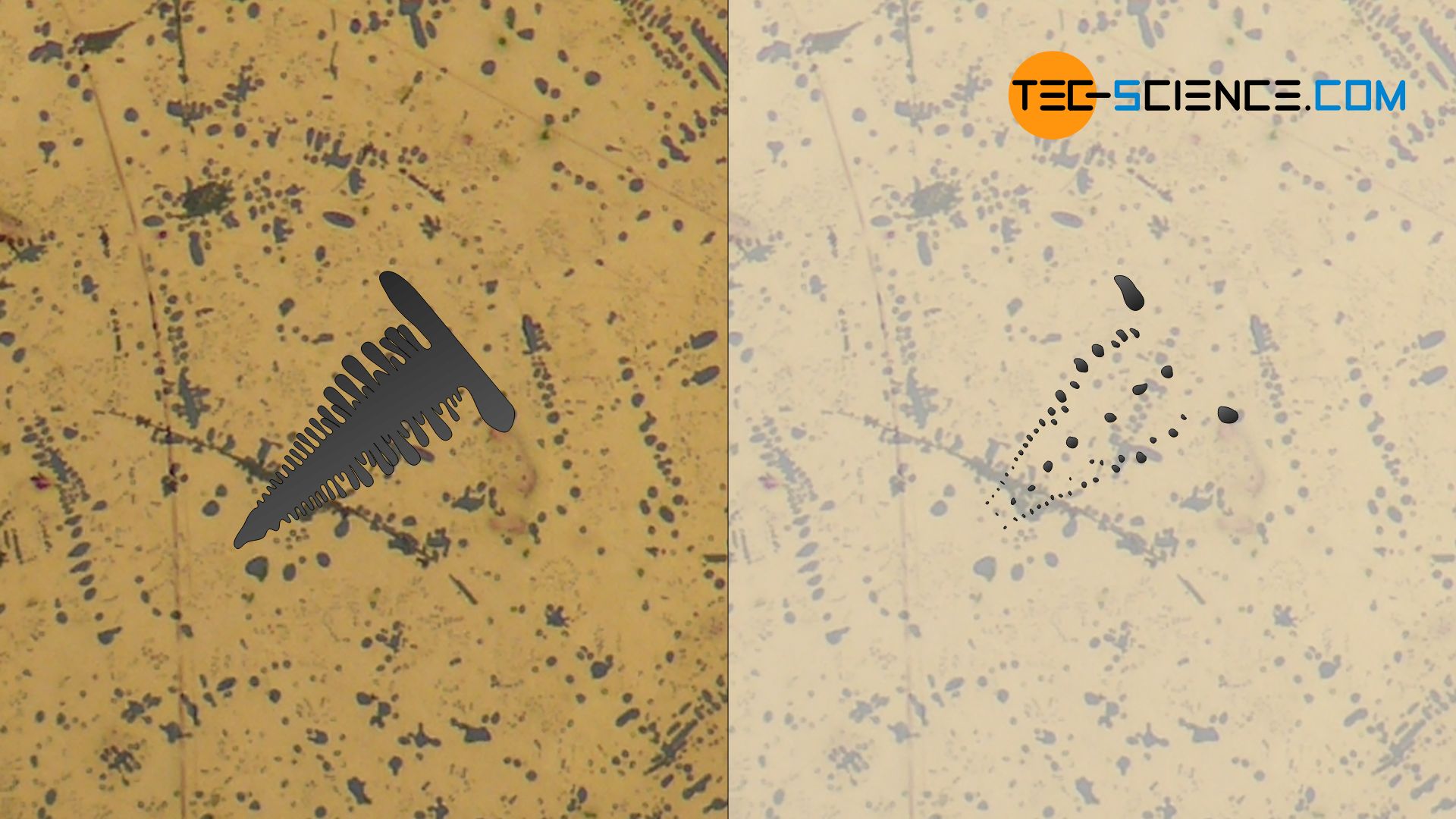
With dendritic crystal growth, there is a fundamental danger that no melt can flow into the gaps between the branches . Small microscopic cavities are then formed, which are called microshrinkage.
Excursus: Snowflake
A dendritic crystal growth also shows water when it crystallises into a snowflake on cold winter days. The hexagonal lattice structure of the ice leads to the typical six main branches of the snowflake, from which several smaller branches branch off.