In amorphous metals, supercooling is so strong that nucleation is suppressed and crystalline structures do not form.
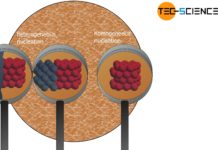
In the article on types of nuclei it was explained that with stronger cooling and thus undercooling, more nuclei will be formed in the melt (high nuclei formation rate). As a result of the many nuclei, a fine-grained and thus a high-strength and tough structure is obtained.
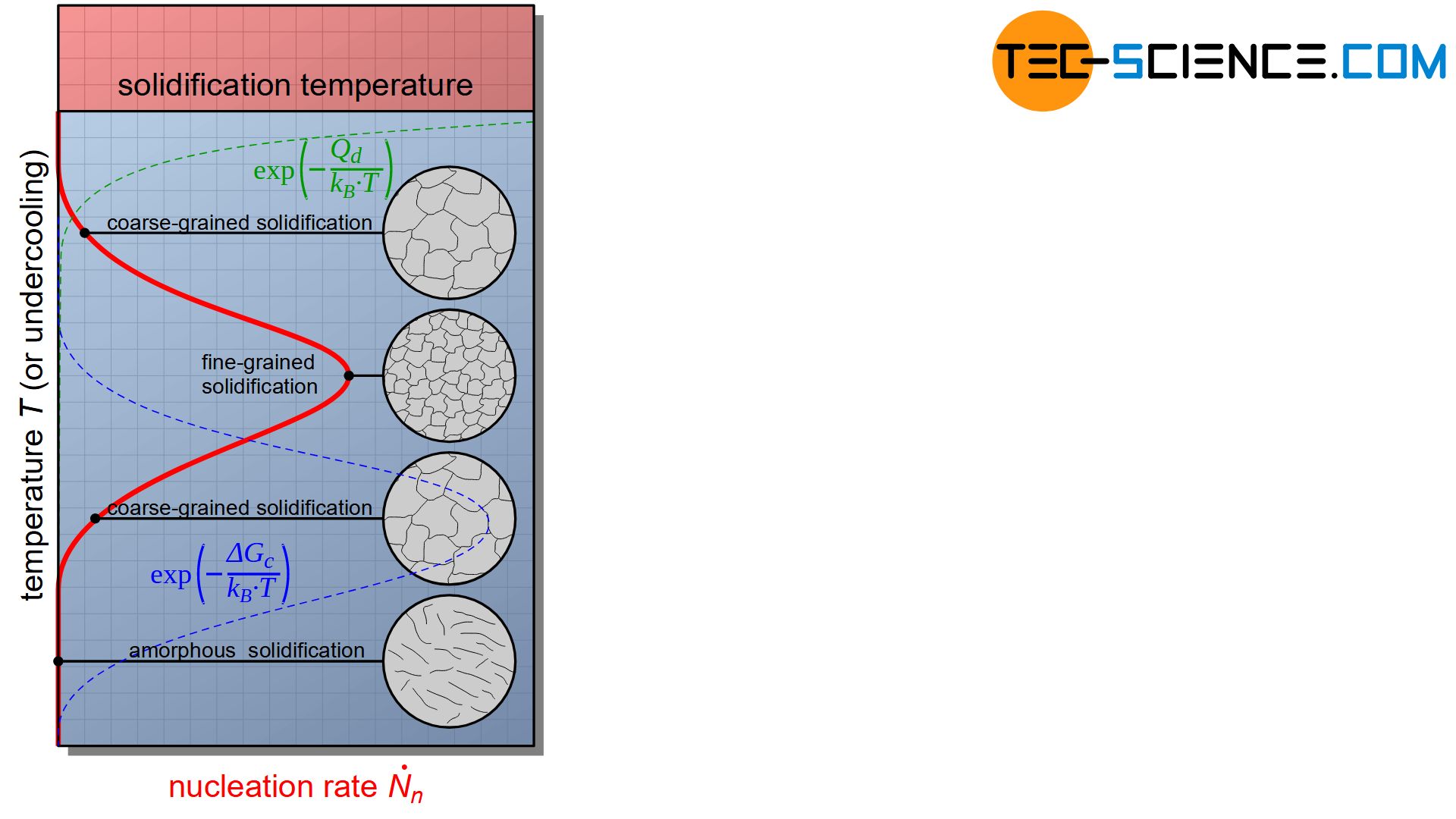
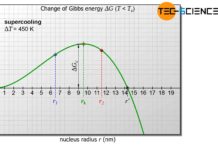
However, there are also limits to such a strong cooling. If it cools down too quickly, the particles do not have time to settle down in a lattice structure. Due to the rapid temperature decrease, the particles become so quickly inert that they can only move to a limited extent. They are practically “frozen” in their molten state.
How quickly a melt must be cooled down to prevent crystallization depends on the chemical properties of the substance. With glass, for example, this condition is achieved by the already low cooling speeds in air. This is also the reason why glass does not usually have a crystalline structure, but belongs to the amorphous substances. However, if glass were to be cooled very slowly and in a controlled manner, glass would also crystallize! From a thermodynamic point of view, glass is therefore a highly supercooled liquid!
However, because the particles are much too inert in this undercooled state, glass seems “solid”. But as the temperature increases more and more, the flow behavior gradually becomes apparent, as the inertia decreases with higher temperature. As is usual for liquids, the glass then gradually begins to melt.
Glasses therefore do not have a fixed melting point (one speaks of a so-called glass transition temperature)! But even at room temperature, a flow of the glass can theoretically be observed. In practice, however, this requires periods of several thousand to millions of years. This is why glass is considered a solid for practical reasons.
In the same way that crystallization in glass can be suppressed by rapid cooling, amorphous solidification of metals can also be achieved if cooling is sufficiently strong. These metals are then called amorphous metals (also called metallic glasses).
The production of such amorphous metals requires cooling rates in the order of 1000 °C within a few milliseconds. Larger components cannot (yet) be produced in this way because the required cooling rates inside the workpiece cannot be achieved.
Amorphous metals are characterized by extremely high hardness and strength and are limited to a few special applications due to the expensive manufacturing process. Among other things, high-quality golf clubs are made of metallic glass.