According to Rutherford’s atomic model, negatively charged electrons move around a positively charged atomic nucleus.
In 1910, the physicist Ernest Rutherford found that when a thin gold foil was bombarded with α-particles (twice positively charged helium nuclei with two neutrons \( ^4_2\text{He}^{2+} \)), only very few of these particles collided with the atomic nuclei of the gold atoms. Almost all α-particles traveled on a straight trajectory through the foil, while only a few were deflected.
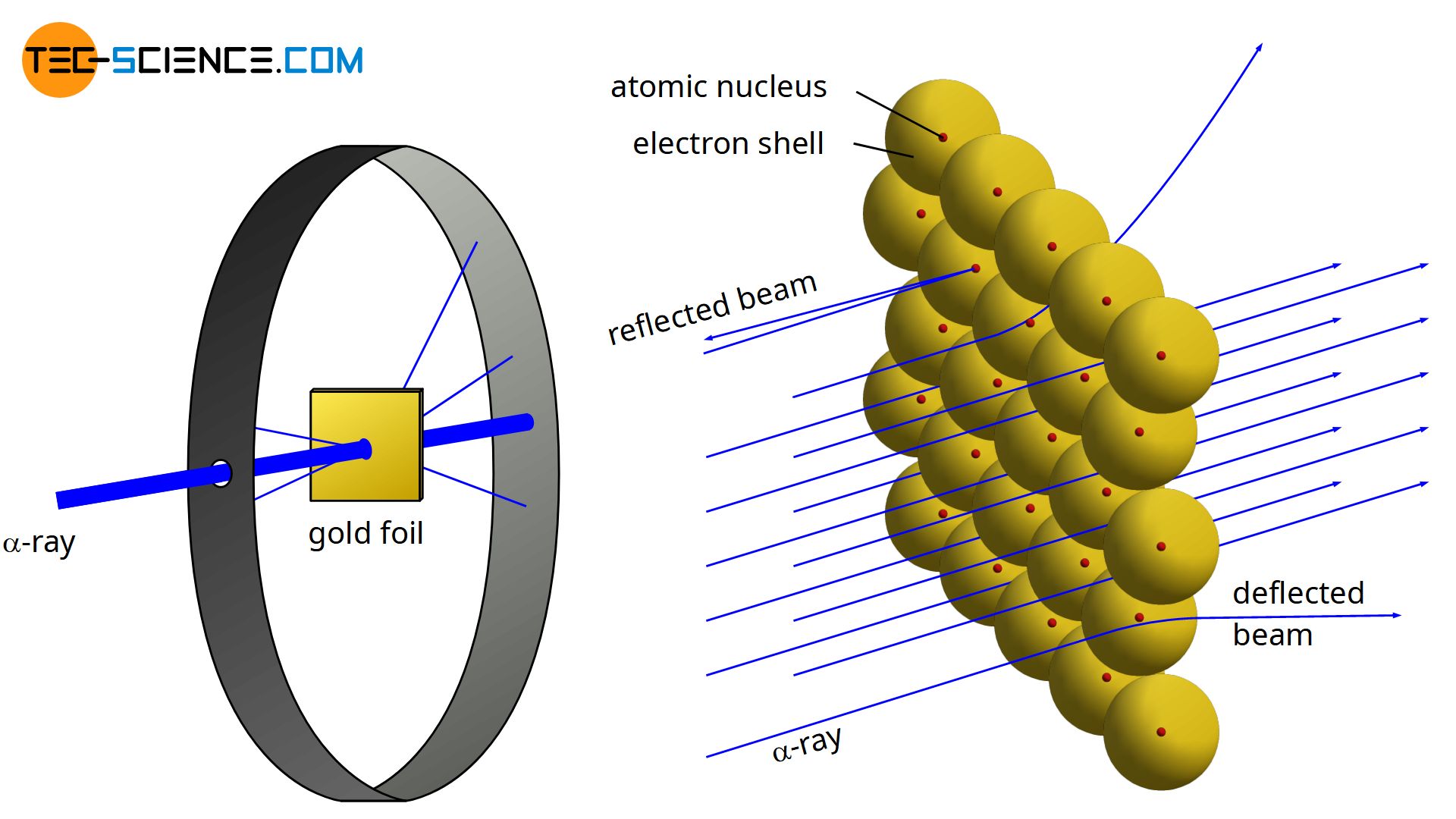
Obviously, very few α-particles were close enough to the positive nucleus of the gold atoms that they could be deflected to a significant degree by the repulsive forces. In most cases, the α-particles traversed the gold foil at quite a distance from the respective atomic nuclei and were scarcely affected in their trajectory. This experiment concluded that the nucleus would have to be much smaller compared to the rest of the atom or rather to its atomic shell.
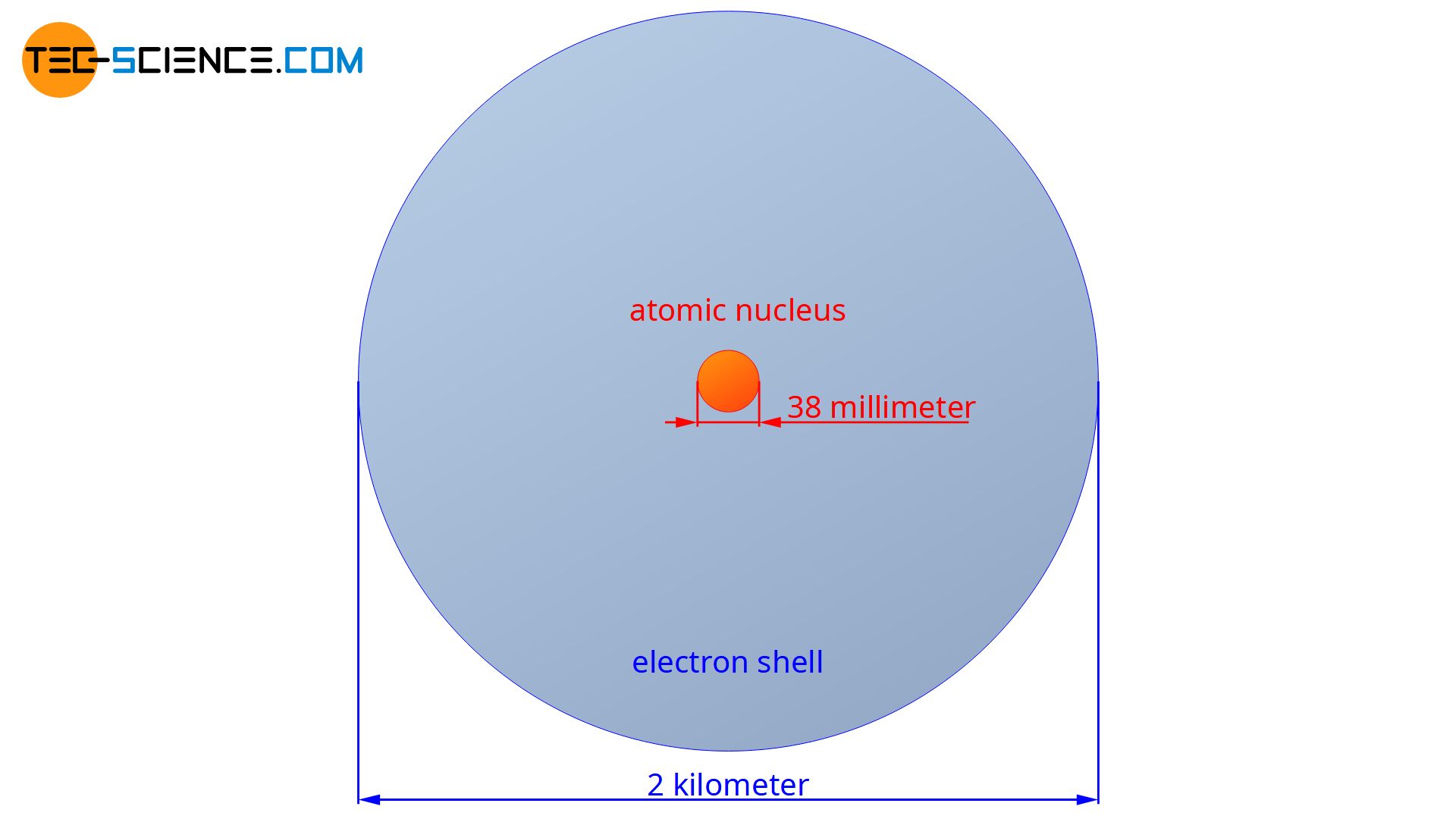
Today we know that the atomic nucleus has a diameter which is 10,000 to 100,000 times smaller than the atomic shell! If the atomic nucleus had the size of a dollar coin, the diameter of the atomic shell would amount to about 2 km!
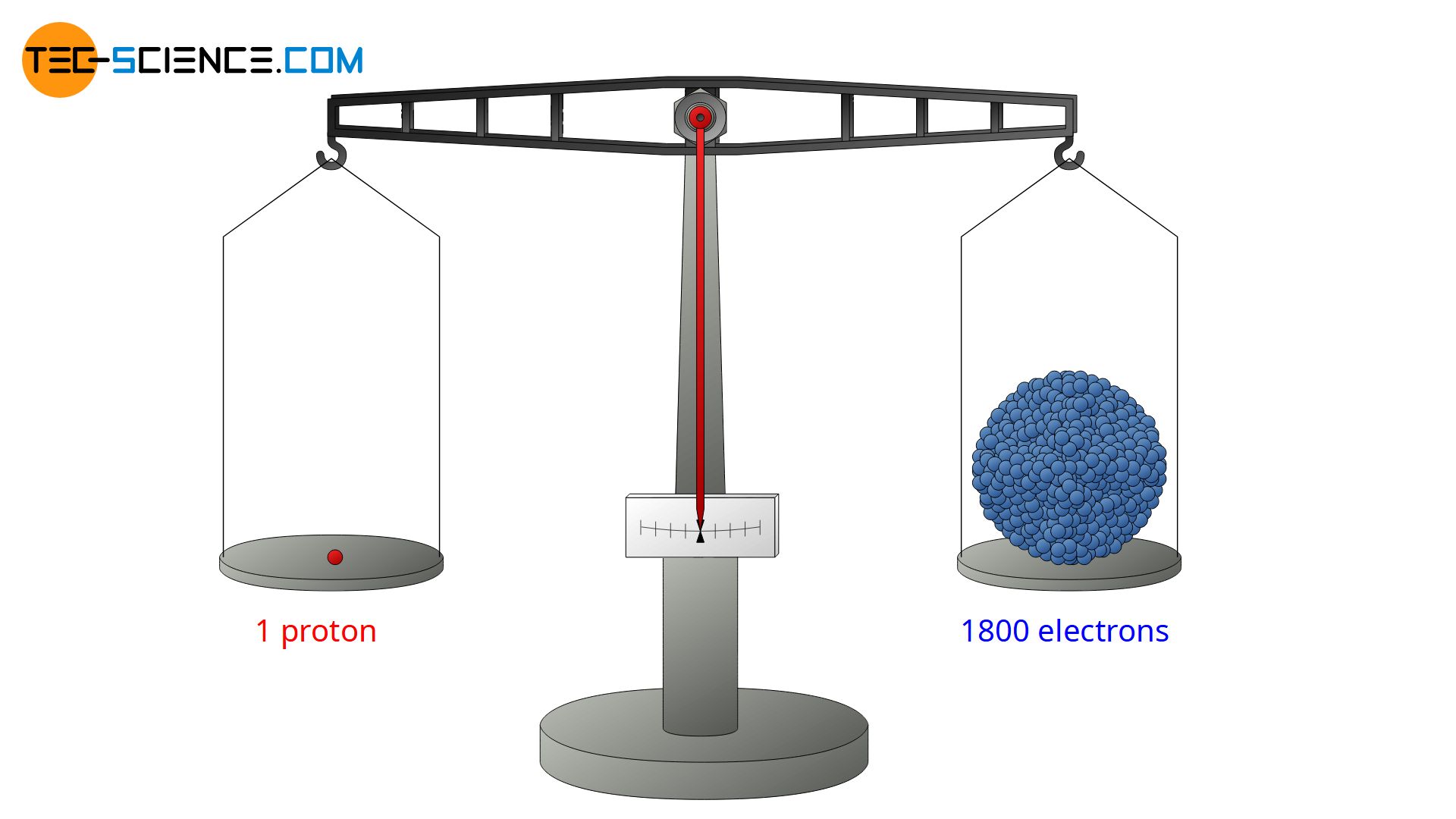
The gold foil experiment further showed that some α-particles were reflected back to the gold foil with almost no energy loss. They obviously had to hit something very massive and heavy (analogous to a tennis ball that hits a massive concrete wall and flies back at almost the same speed). From this, Rutherford concluded that nearly the entire mass of an atom must be concentrated in the nucleus to produce such a strong reflection effect. And indeed, nearly 99.9% of the total mass of an atom is contained in its nucleus. Only 0.1% of the mass is therefore attributable to the atomic shell. Today we know that a proton (as well as a neutron) has a mass about 1800 times as large as an electron.
These findings formed the basis for Rutherford’s atomic model (Rutherford model), whose quintessences are summarized below:
- an atom consists of an atomic nucleus and an atomic shell,
- the nucleus is positively charged and the atomic shell carries a negative charge,
- in the nucleus are positively charged protons (and neutrons),
- in the atomic shell are the negatively charged electrons,
- the nucleus is much smaller than the atomic shell and
- almost the entire mass of an atom is concentrated in its nucleus.
With the Rutherford model, the results of scattering experiments (such as those of the gold foil experiment) could be correctly explained. The basic mass and size ratios as well as the corresponding division into atomic nucleus and electron shell also reflect this atomic model.
For example, the question of why atoms can only be excited with certain energies can not be answered by this model. Or why atoms emit characteristic line spectra. Likewise, Rutherford’s atomic model gives no explanation why an atom is stable, because the circular motion of the electrons around the nucleus would actually lead to an energy dissipation. Accordingly, the electrons ought to fall into the nucleus after only a short time and no atom should therefore be stable!
Some of the weaknesses of Rutherford’s atomic model could be corrected by the physicist Niels Bohr in his model (Bohr model).
Note
In principle, models (such as the atomic models or the particle model) never claim to give a complete explanation of reality. Models are always attempts to depict reality within certain limits and make it explainable.
The Rutherford model is not fundamentally “wrong” but has only limits of validity. Therefore, the Rutherford is not obsolete but it depends on the phenomena to be described and explained. To explain, for example, the gold foil experiment, the Rutherford model is completely sufficient; this does not require an unnecessarily complex quantum mechanical model.
Models are attempts to describe observable phenomena within certain validity limits.