In metal bonding, the metal atoms give off their outer electrons and in this way achieve the noble gas configuration.
The main type of bonding between two metals is so-called metal bond. The metal atoms give off all their valence electrons and thus reach the noble gas configuration.
The metal atoms become positively charged cations upon release of the electrons. Between these positively charged cations, the released electrons form the so-called electron gas, since the electrons can move freely in the atomic structure as in a gas so to speak. The cohesion of the atoms is due to the electrostatic attraction between the positively charged cations and the negatively charged electron gas. As the name implies, this type of binding has a special significance, especially in the case of metals.
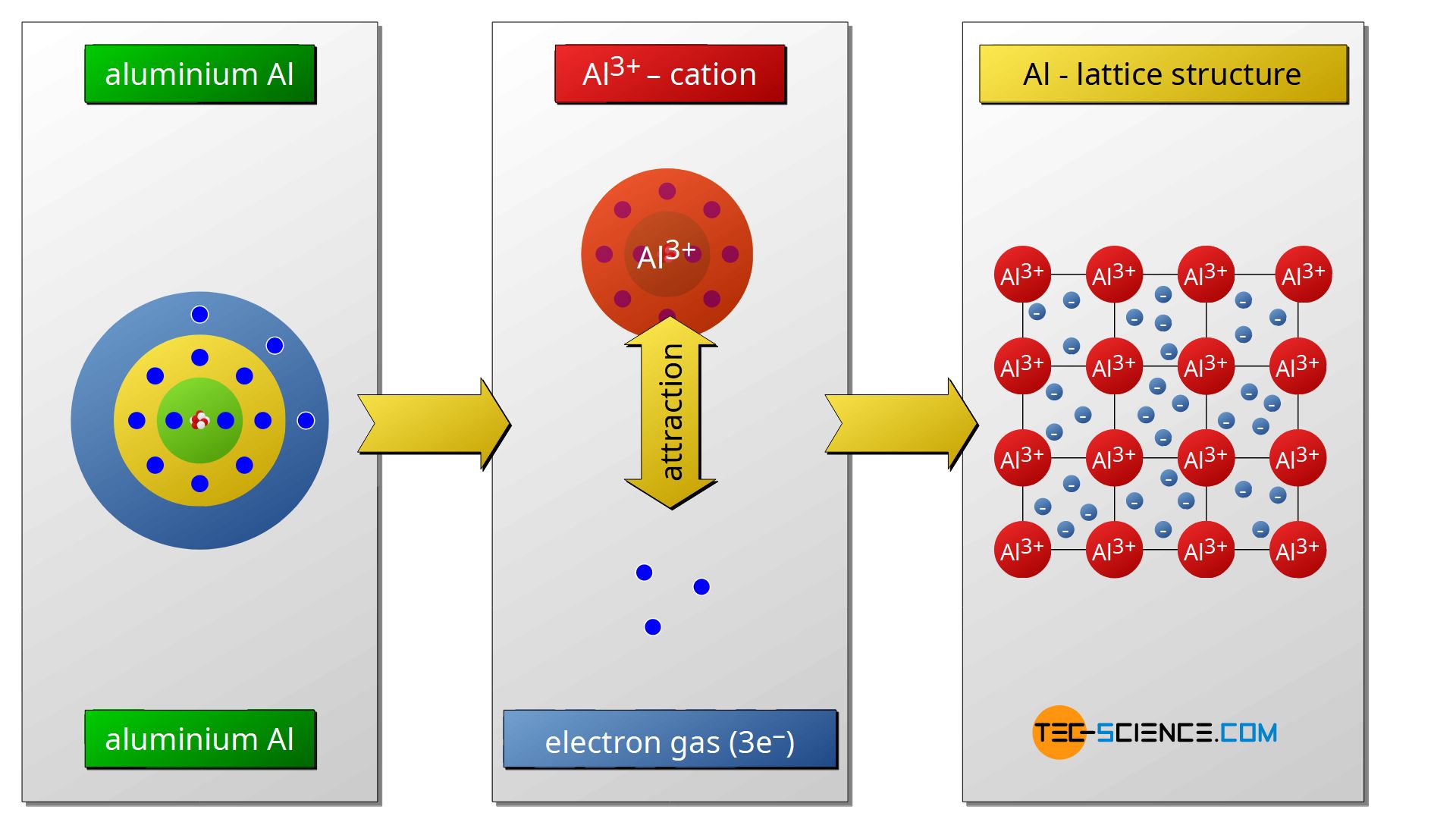
The free mobility of the electrons in the electron gas is ultimately the cause of the generally good electrical and thermal conductivity of metals (the exception to this property is the group of so-called metalloids). The mutual repulsive forces of the metal cations and the simultaneous attracting force of the electron gas lead to a regular lattice structure.
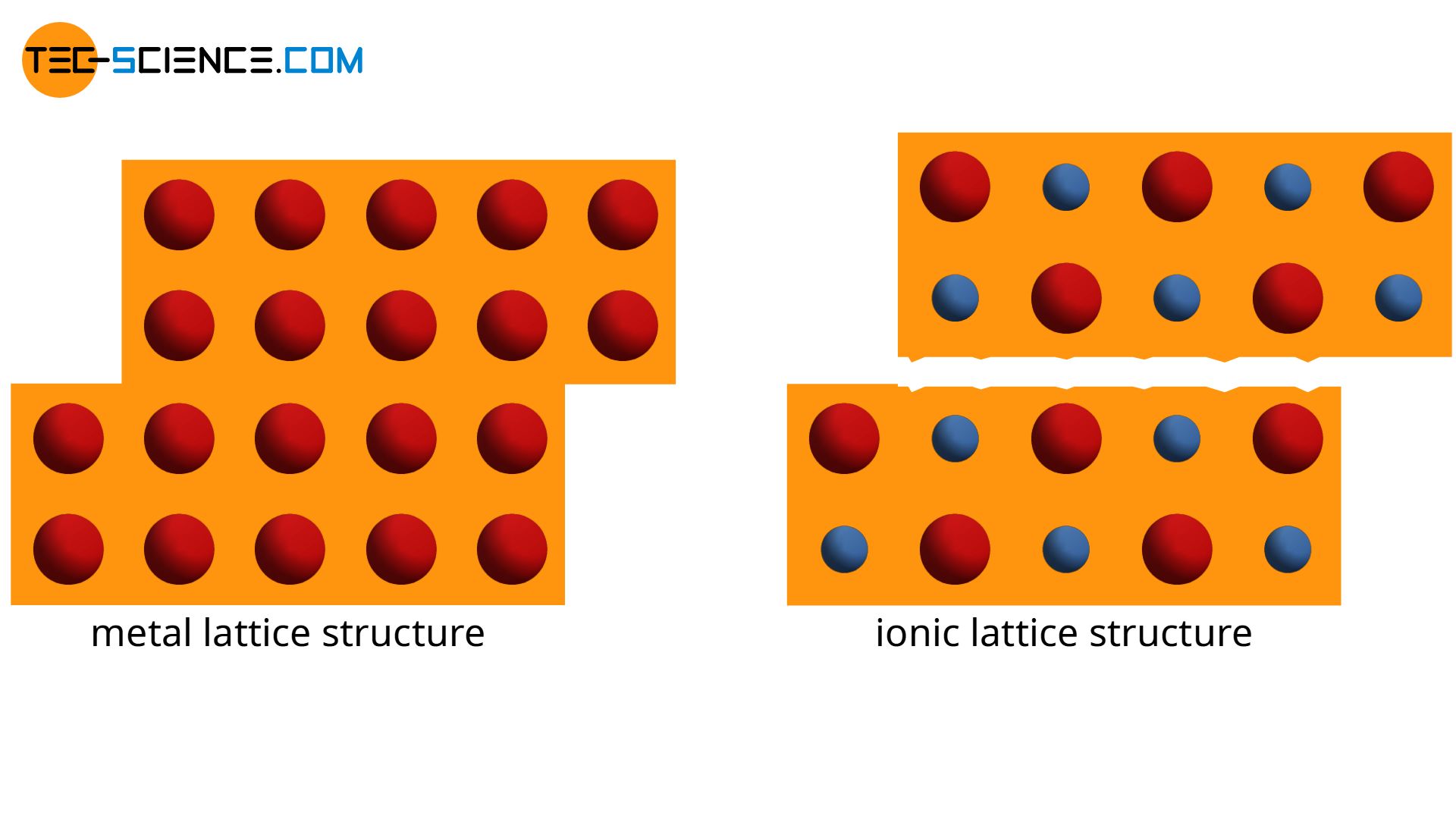
In contrast to the lattice structure of an ionic bond, which consist of anions or cations, the atomic structure of the metal bond is completely identical. When single atoms or entire atomic series are displaced, there are basically no changes in the atomic structure in a metal.
In contrast to this, opposed charged ions encounter one another when shifting the ionic lattice. The repulsive forces between the identical ions finally “shatter” the material. This is the reason why ceramics are much more brittle due to their ionic bonding and can not be deformed like metals.